|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In normal lungs, approximately two thirds of each breath reaches
perfused alveoli to take part in gas exchange. This constitutes the effective or
alveolar ventilation (V̇A). The remaining third
of each breath takes no part in gas exchange and is therefore termed the total (or
physiologic) dead space ventilation (VD). The relationship
is as follows: alveolar ventilation (V̇A)
= frequency (f) (VT − VD).
The physiologic (or total) dead space ventilation (VDphysiologic
)
may be further divided into two components: a volume of gas that ventilates the
conducting airways, the anatomic dead space (VDanatomic
),
and a volume of gas that ventilates unperfused alveoli, the alveolar dead space (VDalveolar
).
Clinical examples of VDalveolar
ventilation
include zone 1, pulmonary embolus, and destroyed alveolar septa, and such ventilation
therefore does not participate in gas exchange. Figure
17-21
shows a two-compartment model of the lung in which the anatomic and
alveolar dead space compartments have been combined into the total (physiologic)
dead space compartment; the other compartment is the alveolar ventilation compartment,
whose idealized V̇A/![]() ratio is 1.0.
ratio is 1.0.
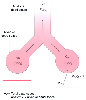 Figure 17-21
Two-compartment model of the lung in which the anatomic
and alveolar dead space compartments have been combined into the total (physiologic)
dead space (VD). FACO2
= alveolar CO2
fraction; FECO2
= mixed expired CO2
fraction; FICO2
= inspired CO2
fraction; V̇A = alveolar
ventilation; V̇A/
Figure 17-21
Two-compartment model of the lung in which the anatomic
and alveolar dead space compartments have been combined into the total (physiologic)
dead space (VD). FACO2
= alveolar CO2
fraction; FECO2
= mixed expired CO2
fraction; FICO2
= inspired CO2
fraction; V̇A = alveolar
ventilation; V̇A/![]() = 1
means ventilation and perfusion are equal in liters per minute; V̇CO2
= carbon dioxide production; V̇E = expired minute
ventilation. Normally, the amount of CO2
eliminated at the airway (V̇E
× FECO2
) equals the amount of CO2
removed by alveolar ventilation (V̇A
× FACO2
) because there
is no CO2
elimination from alveolar dead space (FICO2
= 0).
= 1
means ventilation and perfusion are equal in liters per minute; V̇CO2
= carbon dioxide production; V̇E = expired minute
ventilation. Normally, the amount of CO2
eliminated at the airway (V̇E
× FECO2
) equals the amount of CO2
removed by alveolar ventilation (V̇A
× FACO2
) because there
is no CO2
elimination from alveolar dead space (FICO2
= 0).
The anatomic dead space varies with lung size and is approximately 2 mL/kg of body weight (150 mL in a 70-kg adult). In a normal healthy adult lying supine, anatomic dead space and total (physiologic) dead space are approximately equal to each other because alveolar dead space is normally minimal. In the erect posture, the uppermost alveoli may not be perfused (zone 1), and alveolar dead space may increase from a negligible amount to 60 to 80 mL.
Figure 17-21
illustrates that in a steady state, the volume of CO2
entering the alveoli
(V̇CO2
) is equal to the volume of
CO2
eliminated in the expired gas (V̇E)(FECO2
).
Thus V̇CO2
= (V̇E)(FECO2
).
However, the expired gas volume consists of alveolar gas (V̇A)(FACO2
)
and V̇D gas (V̇D)(FICO2
).
Thus, V̇CO2
= (V̇A)(FACO2
)
+ (V̇D)(FICO2
).
Setting the first equation equal to the second equation and using the relationship
V̇E = V̇A
+ V̇D, subsequent algebraic manipulation,
including setting PACO2
equal to PaCO2
,
results in the modified Bohr equation:
VD/VT
= (PaCO2
− PECO2
)/PaCO2
(9)
The PECO2 value may be obtained by measuring exhaled CO2 in a large (Douglas) bag, or more commonly, end-tidal PCO2 (PETCO2 ) can be used as a surrogate. In severe lung disease, physiologic VD/VT provides a useful expression of
The alveolar concentration of a gas is equal to the difference between the inspired concentration of a gas and the ratio of the output (or uptake) of the gas to V̇A. Thus, for gas X during dry conditions, PAX = (Pdry atm) (FIX) ± V̇X (output or uptake)/V̇A, where PAX = alveolar partial pressure of gas X, FIX = inspired concentration of gas X, Pdry atm = dry atmospheric pressure = Pwet atm − PH2 O = 760 − 47 = 713 mm Hg, V̇X = output or uptake of gas X, and V̇A = alveolar ventilation.
For CO2
, PACO2
= 713(FICO2
+ V̇CO2
/V̇A).
Because FICO2
= 0 and using standard
conversion factors:
PACO2
= 713[V̇CO2
(mL/min STPD)/V̇A (L/min/BTPS)(0.863)] (10)
 Figure 17-22
Relationship between minute ventilation (V̇E,
L/min) and PaCO2
for a family of ratios
of total dead space to tidal volume (VD/VT).
These curves are hyperbolic (see Equation 10) and
rise steeply at low VE values.
Figure 17-22
Relationship between minute ventilation (V̇E,
L/min) and PaCO2
for a family of ratios
of total dead space to tidal volume (VD/VT).
These curves are hyperbolic (see Equation 10) and
rise steeply at low VE values.
For O2
,
PAO2
= 713[FIO2
− V̇O2
(mL/min)/V̇A
(mL/min)] (11)
For example, 100 mm Hg = 713(0.21 − 225/3200).
Figure 17-23 shows the hyperbolic relationships expressed in Equations 10 and 11 between PaCO2 and V̇A (see Fig. 17-22 ) and between PAO2 and V̇A for different levels of V̇CO2 and V̇O2 , respectively. PaCO2 is substituted for PACO2 because PACO2 -to-PaCO2 gradients are small (as opposed to PAO2 -to-PaO2 gradients, which can be large). Note that as V̇A increases, the second term of the right side of Equations 10 and 11 approaches zero and the composition of the alveolar gas approaches that of the inspired gas. In addition, it should be noted from Figure 17-22 , Figure 17-23 , and Figure 17-24 that because anesthesia is usually administered with an oxygen-enriched gas mixture, hypercapnia is a more common result of hypoventilation than hypoxemia is.
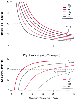 Figure 17-23
The relationship between alveolar ventilation and PAO2
and PaO2
for a group of different O2
consumption (V̇O2
) and CO2
production (V̇CO2
) values is derived
from Equations 10 and 11 in the text and is hyperbolic. As alveolar ventilation
increases, PAO2
and PaCO2
approach inspired concentrations. Decreases in alveolar ventilation to less than
4 L/min are accompanied by precipitous decreases in PAO2
and increases in PaCO2
.
Figure 17-23
The relationship between alveolar ventilation and PAO2
and PaO2
for a group of different O2
consumption (V̇O2
) and CO2
production (V̇CO2
) values is derived
from Equations 10 and 11 in the text and is hyperbolic. As alveolar ventilation
increases, PAO2
and PaCO2
approach inspired concentrations. Decreases in alveolar ventilation to less than
4 L/min are accompanied by precipitous decreases in PAO2
and increases in PaCO2
.
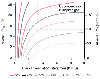 Figure 17-24
For any given O2
concentration in inspired
gas, the relationship between alveolar ventilation and PAO2
is hyperbolic. As the inspired O2
concentration is increased, the amount
that alveolar ventilation must decrease to produce hypoxemia is greatly increased.
BTPS, body temperature, ambient pressure, saturated. (Redrawn from Lumb
AB: Respiratory system resistance: Measurement of closing capacity. In
Lumb AB [ed]: Nunn's Applied Respiratory Physiology, 5th ed. London, Butterworths,
2000, p 79.)
Figure 17-24
For any given O2
concentration in inspired
gas, the relationship between alveolar ventilation and PAO2
is hyperbolic. As the inspired O2
concentration is increased, the amount
that alveolar ventilation must decrease to produce hypoxemia is greatly increased.
BTPS, body temperature, ambient pressure, saturated. (Redrawn from Lumb
AB: Respiratory system resistance: Measurement of closing capacity. In
Lumb AB [ed]: Nunn's Applied Respiratory Physiology, 5th ed. London, Butterworths,
2000, p 79.)
The principal function of the heart and lungs is supporting O2
delivery to and CO2
removal from the tissues in accordance with metabolic
requirements while maintaining arterial blood O2
and CO2
partial
pressures within a narrow range. The respiratory and cardiovascular systems are
series-linked to accomplish this function over a wide range of metabolic requirements,
which may increase 30-fold from rest to heavy exercise. The functional links in
the oxygen transport chain are as follows: (1) ventilation and distribution of ventilation
with respect to perfusion, (2) diffusion of O2
into blood,
| Link in Chain | Functional Capacity in Normal Humans | Theoretical Maximum O2 Transport Capacity |
|---|---|---|
| Ventilation | 200 L/min (MVV) | 0.030 × MVV = 6.0 L O2 /min |
| Diffusion and chemical reaction | DLO2 = 6.1 L O2 /min | |
| CO | 20 L/min |
|
| O2 extraction | 75% | 0.16 × CO = 3.2 L O2 /min |
| (a-v O2 difference) | (16 mL O2 /100 mL or 0.16) |
|
| CO = cardiac output; MVV = maximum voluntary ventilation. | ||
| From Cassidy SS: Heart-lung interactions in health and disease. Am J Med Sci 30:451–461, 1987. | ||
The maximum functional capacity of each link can be determined independently. Table 17-3 lists these measured functional capacities for healthy, young men. Because theoretical maximal O2 transport at the ventilatory step or the diffusion and chemical reaction steps (about 6 L/min in healthy humans at sea level) exceeds the O2 transportable by the maximum cardiac output and distribution steps, the limit to O2 transport is the cardiovascular system. Respiratory diseases would not be expected to limit maximum O2 transport until functional capacities are reduced nearly 40% to 50%.
As a red blood cell (RBC) passes by the alveolus, O2 diffuses into plasma and increases PaO2 . As PaO2 increases, O2 diffuses into the RBC and combines with Hb. Each Hb molecule consists of four heme molecules attached to a globin molecule. Each heme molecule consists of glycine, α-ketoglutaric acid, and iron in the ferrous (Fe2+ ) form. Each ferrous ion has the capacity to bind with one O2 molecule in a loose, reversible combination. As the ferrous ions bind to O2 , the Hb molecule begins to become saturated.
The oxy-Hb dissociation curve relates the saturation of Hb (rightmost y axis in Fig. 17-25 ) to PaO2 . Hb is fully saturated (100%) by a PO2 of about 700 mm Hg. The normal arterial point on the right side and flat part of the oxy-Hb curve in Figure 17-25 is 95% to 98% saturated by a PaO2 of about 90 to 100 mm Hg. When PO2 is less than 60 mm Hg (90% saturation), saturation falls steeply, and the amount of Hb uncombined with O2 increases greatly for a given decrease in PO2 . Mixed venous blood has a PO2 (Pv̄O2 ) of about 40 mm Hg and is approximately 75% saturated, as indicated by the middle of the three points on the oxy-Hb curve in Figure 17-25 .
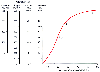 Figure 17-25
Oxygen-hemoglobin dissociation curve. Four different
ordinates are shown as a function of oxygen partial pressure (the abscissa). In
order from right to left, they are saturation (%); O2
content (mL of O2
/0.1
L of blood); O2
supply to peripheral tissues (mL/min); and O2
available to peripheral tissues (mL/min), which is O2
supply minus the
approximately 200 mL/min that cannot be extracted below a partial pressure of 20
mm Hg. Three points are shown on the curve: a, normal arterial pressure;
Figure 17-25
Oxygen-hemoglobin dissociation curve. Four different
ordinates are shown as a function of oxygen partial pressure (the abscissa). In
order from right to left, they are saturation (%); O2
content (mL of O2
/0.1
L of blood); O2
supply to peripheral tissues (mL/min); and O2
available to peripheral tissues (mL/min), which is O2
supply minus the
approximately 200 mL/min that cannot be extracted below a partial pressure of 20
mm Hg. Three points are shown on the curve: a, normal arterial pressure; ![]() ,
normal mixed venous pressure; and P50
, the partial pressure (27 mm Hg)
at which hemoglobin is 50% saturated.
,
normal mixed venous pressure; and P50
, the partial pressure (27 mm Hg)
at which hemoglobin is 50% saturated.
The oxy-Hb curve can also relate the O2
content (CO2
)
(vol%, mL of O2
/0.1 L of blood; second most right y
axis in Fig. 17-25
) to PO2
.
Oxygen is carried in solution in plasma, 0.003 mL of O2
/mm Hg PO2
/0.1
L, and is combined with Hb, 1.39 mL of O2
/g of Hb, to the extent (percentage)
that Hb is saturated. Thus,
CO2
= (1.39)(Hb)(percent
saturation) + 0.003(PO2
) (12)
For a patient with an Hb content of 15 g/0.1 L, PaO2
of 100 mm Hg, and Pv̄O2
of 40 mm
Hg, the arterial O2
content (CaO2
)
= (1.39)(15)(1) + (0.003)(100) = 20.9 + 0.3 = 21.2
mL of O2
/0.1 L; the mixed venous O2
content (Cv̄O2
)
= (1.39) (15)(0.75) + (0.003)(40) = 15.6 + 0.1 = 15.7
mL of O2
/0.1 L. Thus, the normal arteriovenous O2
content difference is approximately 5.5 mL/0.1 L.
Note that Equation 12 uses the constant 1.39, which means that 1 g of Hb can carry 1.39 mL of oxygen. Controversy exists over the magnitude of this number. Originally, 1.34 had been used,[95] but with determination of the molecular weight of Hb (64,458), the theoretical value of 1.39 became popular.[96] After extensive human studies, Gregory[97] observed in 1974 that the applicable value was 1.31 mL O2 /g of Hb in human adults. The reason that the clinically measured CO2 is lower than the theoretical 1.39 is probably due to the small amount of methemoglobin (Met-Hb) and carboxyhemoglobin (CO-Hb) normally present in blood.
The oxy-Hb curve can also relate O2
transport (L/min)
to the peripheral tissues (see the third most right y
axis in Fig. 17-25
) to PO2
.
This value is obtained by multiplying the O2
content by ![]() T
(O2
transport =
T
(O2
transport = ![]() T
×CaO2
). To do this multiplication,
one must convert the content unit of mL/0.1 L to mL/L by multiplying the usual O2
content by 10 (results in mL of O2
/L of blood); subsequent multiplication
of mL/L against
T
×CaO2
). To do this multiplication,
one must convert the content unit of mL/0.1 L to mL/L by multiplying the usual O2
content by 10 (results in mL of O2
/L of blood); subsequent multiplication
of mL/L against ![]() T in L/min yields mL/min.
Thus, if
T in L/min yields mL/min.
Thus, if ![]() T = 5 L/min and CaO2
= 20 mL of O2
/0.1 L, the arterial point corresponds to 1000 mL O2
/min
going to the periphery, and the venous point corresponds to 750 mL O2
/min
returning to the lungs, with V̇O2
= 250 mL/min.
T = 5 L/min and CaO2
= 20 mL of O2
/0.1 L, the arterial point corresponds to 1000 mL O2
/min
going to the periphery, and the venous point corresponds to 750 mL O2
/min
returning to the lungs, with V̇O2
= 250 mL/min.
The oxy-Hb curve can also relate the O2
actually available
to the tissues (see leftmost y axis in Fig.
17-25
) as a function of PO2
.
Of the 1000 mL/min of O2
normally going to the periphery, 200 mL/min
of O2
cannot be extracted because it would lower PO2
below the level (see the rectangular dashed line in Fig.
17-25
) at which organs such as the brain can survive; the O2
available to tissues is therefore 800 mL/min. This amount is approximately three
to four times the normal resting V̇O2
.
When ![]() T = 5 L/min and arterial saturation is
less than 40%, the total flow of O2
to the periphery is reduced to 400
mL/min; the available O2
is now 200 mL/min and O2
supply just
equals O2
demand. Consequently, with low arterial saturation, tissue
demand can be met only by an increase in
T = 5 L/min and arterial saturation is
less than 40%, the total flow of O2
to the periphery is reduced to 400
mL/min; the available O2
is now 200 mL/min and O2
supply just
equals O2
demand. Consequently, with low arterial saturation, tissue
demand can be met only by an increase in ![]() T
or, in the longer term, by an increase in Hb concentration.
T
or, in the longer term, by an increase in Hb concentration.
The affinity of Hb for O2 is best described by the PO2 level at which Hb is 50% saturated (P50 ) on the oxy-Hb curve. The normal adult P50 is 26.7 mm Hg (see the point on the left side and steep portion of the oxy-Hb curve in Fig. 17-24 ).
The effect of a change in PO2 on Hb saturation is related to both P50 and the portion of the oxy-Hb curve at which the change occurs.[98] In the region of normal PaO2 (75 to 100 mm Hg), the curve is relatively horizontal, so shifts of the curve have little effect on saturation. In the region of mixed venous PO2 , where the curve is relatively steep, a shift of the curve leads to a much greater
A P50 higher than 27 mm Hg describes a right-shifted oxy-Hb curve, which means that at any given PO2 , Hb has a low affinity for O2 and is less saturated than normal. This higher P50 may allow a lower tissue perfusion than normal to produce the normal amount of O2 unloading. Causes of a right-shifted oxy-Hb curve are acidosis (metabolic and respiratory—the Bohr effect), hyperthermia, abnormal Hb, increased RBC 2,3-DPG content, and inhaled anesthetics (see later).[98]
Abnormalities in acid-base balance result in alteration of 2,3-DPG metabolism to shift the oxy-Hb curve to its normal position. This compensatory change in 2,3-DPG requires between 24 and 48 hours. Thus, with acute acid-base abnormalities, O2 affinity and the position of the oxy-Hb curve change. However, with more prolonged acid-base changes, the reciprocal changes in 2,3-DPG levels shift the oxy-Hb curve and therefore O2 affinity back toward normal.[98]
Many inhaled anesthetics have been shown to shift the oxy-Hb dissociation curve to the right.[99] Isoflurane shifts P50 to the right by 2.6 ± 0.07 mm Hg at a vapor pressure of approximately 1 minimum alveolar concentration (MAC) (1.25%).[100] On the other hand, high-dose fentanyl, morphine, and meperidine do not alter the position of the curve.
PAO2
is directly related
to FIO2
in normal patients. PAO2
and FIO2
also correspond to PaO2
when there is little to no right-to-left transpulmonary shunt (![]() S/
S/![]() T).
Figure 17-26
shows the
relationship between FIO2
and PaO2
for a family of right-to-left transpulmonary shunts (
T).
Figure 17-26
shows the
relationship between FIO2
and PaO2
for a family of right-to-left transpulmonary shunts (![]() S/
S/![]() T);
the calculations assume a constant and normal
T);
the calculations assume a constant and normal ![]() T
and PaCO2
. With no
T
and PaCO2
. With no ![]() S/
S/![]() T,
a linear increase in FIO2
results in a
linear increase in PaO2
(solid straight
line). As the shunt is increased, the
T,
a linear increase in FIO2
results in a
linear increase in PaO2
(solid straight
line). As the shunt is increased, the ![]() S/
S/![]() T
lines relating FIO2
to PaO2
become progressively flatter.[101]
With a shunt
of 50% of QT, an increase in FIO2
results in almost no increase in PaO2
.
The solution to the problem of hypoxemia secondary to a large shunt is not increasing
the FIO2
, but rather causing a reduction
in the shunt (fiberoptic bronchoscopy, PEEP, patient positioning, antibiotics, suctioning,
diuretics).
T
lines relating FIO2
to PaO2
become progressively flatter.[101]
With a shunt
of 50% of QT, an increase in FIO2
results in almost no increase in PaO2
.
The solution to the problem of hypoxemia secondary to a large shunt is not increasing
the FIO2
, but rather causing a reduction
in the shunt (fiberoptic bronchoscopy, PEEP, patient positioning, antibiotics, suctioning,
diuretics).
In addition to an increased ![]() S/
S/![]() T,
CaO2
is decreased by decreased
T,
CaO2
is decreased by decreased ![]() T
(for a constant V̇O2
) and by increased
V̇O2
(for a constant
T
(for a constant V̇O2
) and by increased
V̇O2
(for a constant ![]() T).
In either case (decreased
T).
In either case (decreased ![]() T or
T or
 Figure 17-26
Effect of changes in inspired oxygen concentration on
PaO2
for various right-to-left transpulmonary
shunts. Cardiac output (
Figure 17-26
Effect of changes in inspired oxygen concentration on
PaO2
for various right-to-left transpulmonary
shunts. Cardiac output (![]() T), hemoglobin (Hb),
oxygen consumption (V̇O2
), and arteriovenous
oxygen content differences [C(a - v)O2
]
are assumed to be normal.
T), hemoglobin (Hb),
oxygen consumption (V̇O2
), and arteriovenous
oxygen content differences [C(a - v)O2
]
are assumed to be normal.
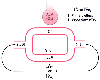 Figure 17-27
Effect of a decrease in cardiac output or an increase
in oxygen consumption on mixed venous and arterial oxygen content. Mixed venous
blood (
Figure 17-27
Effect of a decrease in cardiac output or an increase
in oxygen consumption on mixed venous and arterial oxygen content. Mixed venous
blood (![]() ) either perfuses ventilated alveolar (ALV O2
) capillaries
and becomes oxygenated end-pulmonary capillary blood (c') or perfuses whatever true
shunt pathways exist and remains the same in composition (desaturated). These two
pathways must ultimately join together to form mixed arterial (a) blood. If cardiac
output (
) either perfuses ventilated alveolar (ALV O2
) capillaries
and becomes oxygenated end-pulmonary capillary blood (c') or perfuses whatever true
shunt pathways exist and remains the same in composition (desaturated). These two
pathways must ultimately join together to form mixed arterial (a) blood. If cardiac
output (![]() T) decreases or oxygen consumption
(V̇O2
) increases, or both the tissues
must extract more oxygen per unit volume of blood than under normal conditions.
Thus, the primary effect of a decrease in
T) decreases or oxygen consumption
(V̇O2
) increases, or both the tissues
must extract more oxygen per unit volume of blood than under normal conditions.
Thus, the primary effect of a decrease in ![]() T
or an increase in V̇O2
is a decrease
in mixed venous oxygen content. The mixed venous blood with a decreased oxygen content
must flow through the shunt pathway as before (which may remain constant in size)
and lower the arterial content of oxygen. Thus, the secondary effect of a decrease
in
T
or an increase in V̇O2
is a decrease
in mixed venous oxygen content. The mixed venous blood with a decreased oxygen content
must flow through the shunt pathway as before (which may remain constant in size)
and lower the arterial content of oxygen. Thus, the secondary effect of a decrease
in ![]() T or an increase in V̇O2
is a decrease in arterial oxygen content.
T or an increase in V̇O2
is a decrease in arterial oxygen content.
The amount of O2
flowing through any given particular
lung channel per minute, as depicted in Figure
17-27
, is a product of blood flow times the O2
content of that
blood. Thus, from Figure 17-27
,
![]() T × CaO2
=
T × CaO2
= ![]() c' × Cc'O2
+
c' × Cc'O2
+
![]() S × Cv̄O2
.
With
S × Cv̄O2
.
With ![]() c' =
c' = ![]() T −
T −
![]() S and further algebraic manipulation,
[102]
S and further algebraic manipulation,
[102]
![]() S/
S/![]() T
= Cc'O2
− CaO2
/Cc'O2
− Cv̄O2
(13)
T
= Cc'O2
− CaO2
/Cc'O2
− Cv̄O2
(13)
The larger the intrapulmonary shunt, the greater is the decrease
in CaO2
because more venous blood with
lower Cv̄O2
can admix with end-pulmonary
capillary blood (see Fig. 17-37
).
[103]
[104]
Thus,
P(A - a)O2
is a function both of the size of the ![]() S/
S/![]() T
and of what is flowing through the
T
and of what is flowing through the ![]() S/
S/![]() T,
namely, Cv̄O2
, and Cv̄O2
is a primary function of
T,
namely, Cv̄O2
, and Cv̄O2
is a primary function of ![]() T and V̇O2
.
T and V̇O2
.
Figure 17-28
shows the equivalent circuit of the pulmonary circulation in a patient with a 50%
shunt, a normal Cv̄O2
of 15 vol%,
and a moderately low CaO2
of 17.5 vol%.
Decreasing ![]() T or increasing V̇O2
,
or both, causes a larger primary decrease in Cv̄O2
to 10 vol% and a smaller, but still significant secondary decrease in CaO2
to 15 vol%; the ratio of change in Cv̄O2
to CaO2
in this example of 50%
T or increasing V̇O2
,
or both, causes a larger primary decrease in Cv̄O2
to 10 vol% and a smaller, but still significant secondary decrease in CaO2
to 15 vol%; the ratio of change in Cv̄O2
to CaO2
in this example of 50% ![]() S/
S/![]() T
is 2:1.
T
is 2:1.
 Figure 17-28
The equivalent circuit of the pulmonary circulation in
a patient with a 50% right-to-left shunt. Oxygen content is in mL/100 mL of blood
(vol%). A decrease in cardiac output (
Figure 17-28
The equivalent circuit of the pulmonary circulation in
a patient with a 50% right-to-left shunt. Oxygen content is in mL/100 mL of blood
(vol%). A decrease in cardiac output (![]() T) or
an increase in O2
consumption (V̇O2
)
can cause a decrease in mixed venous oxygen content (from 15 to 10 vol% in this example),
which in turn will cause a decrease in the arterial content of oxygen (from 17.5
to 15.0 vol%). In this 50% shunt example, the decrease in mixed venous oxygen content
was twice the decrease in arterial oxygen content.
T) or
an increase in O2
consumption (V̇O2
)
can cause a decrease in mixed venous oxygen content (from 15 to 10 vol% in this example),
which in turn will cause a decrease in the arterial content of oxygen (from 17.5
to 15.0 vol%). In this 50% shunt example, the decrease in mixed venous oxygen content
was twice the decrease in arterial oxygen content.
If a decrease in ![]() T or an
increase in V̇O2
is accompanied by
a decrease in
T or an
increase in V̇O2
is accompanied by
a decrease in ![]() S/
S/![]() T,
there may be no change in PaO2
(a decreasing
effect on PaO2
is offset by an increasing
effect on PaO2
) ( Table
17-4
). These changes sometimes occur in diffuse lung disease. If a decrease
in
T,
there may be no change in PaO2
(a decreasing
effect on PaO2
is offset by an increasing
effect on PaO2
) ( Table
17-4
). These changes sometimes occur in diffuse lung disease. If a decrease
in ![]() T or an increase in V̇O2
is accompanied by an increase in
T or an increase in V̇O2
is accompanied by an increase in ![]() S/
S/![]() T,
PaO2
may be greatly decreased (a decreasing
effect on PaO2
is compounded by another
decreasing effect on PaO2
) (see Table
17-4
). These changes sometimes occur in regional ARDS and atelectasis.
[105]
T,
PaO2
may be greatly decreased (a decreasing
effect on PaO2
is compounded by another
decreasing effect on PaO2
) (see Table
17-4
). These changes sometimes occur in regional ARDS and atelectasis.
[105]
The Fick principle allows calculation of V̇O2
and states that the amount of O2
consumed by the body (V̇O2
)
is equal to the amount of O2
leaving the lungs (![]() T)(CaO2
)
minus the amount of O2
returning to the lungs (
T)(CaO2
)
minus the amount of O2
returning to the lungs (![]() T)(CEO2
).
Thus
T)(CEO2
).
Thus
V̇O2
=
(![]() T)(CaO2
)
− (
T)(CaO2
)
− (![]() T(Cv̄O2
=
T(Cv̄O2
= ![]() T(CaO2
− Cv̄O2
)
T(CaO2
− Cv̄O2
)
Condensing the content symbols yields the usual expression of the Fick equation:
V̇O2
=
(![]() T[C(a − v)O2
]
(14)
T[C(a − v)O2
]
(14)
This equation states that O2
consumption is equal to ![]() T
times the arteriovenous O2
content difference. Normally, (5
L/min)(5.5 mL)/0.1 L = 0.27 L/min (see the section "Oxygen-Hemoglobin
Dissociation Curve").
T
times the arteriovenous O2
content difference. Normally, (5
L/min)(5.5 mL)/0.1 L = 0.27 L/min (see the section "Oxygen-Hemoglobin
Dissociation Curve").
V̇O2
=
V̇E(FIO2
)
− V̇E(FEO2
)
= V̇E(FIO2
− FEO2
) (15)
Similarly, the amount of O2
consumed by the body (V̇O2
)
is equal to the amount of O2
brought into the lungs by ventilation (V̇I)(FIO2
)
minus the amount of O2
leaving the lungs by ventilation (V̇E)(FEO2
),
where V̇E is expired minute ventilation and FEO2
is the mixed expired O2 fraction.
Thus, V̇O2 = (V̇I)(FIO2 ) − (V̇E(FEO2 ). Because the difference between V̇I and V̇E is due to the difference between V̇O2 (normally 250 mL/min) and V̇CO2 (normally 200 mL/min) and is only 50 mL/min (see later), V̇I essentially equals V̇E.
Normally, V̇O2
= 5.0 L/min (0.21 − 0.16) = 0.25 L/min. In determining
V̇O2
in this way, V̇E
can be measured with a spirometer, FIO2
can be measured with an O2
analyzer or from known fresh gas flows, and
FEO2
can be measured by collecting expired
gas in a bag for a few minutes.
| Changes | Clinical Situation |
|---|---|
|
If |
Decreased cardiac output, stable shunt |
|
If |
Application of PEEP in ARDS |
|
If |
Shock combined with ARDS or atelectasis |
| ARDS, adult respiratory distress syndrome; K, constant; PEEP, positive end expiratory pressure; ↓, decrease; ↑, increase. | |
Additionally, the Fick equation is useful in understanding the
impact of changes in ![]() T on PaO2
and Pv̄O2
. If
T on PaO2
and Pv̄O2
. If ![]() O2
remains constant (K) and
O2
remains constant (K) and ![]() T decreases (↓),
the arteriovenous O2
content difference has to increase (↑):
T decreases (↓),
the arteriovenous O2
content difference has to increase (↑):
V̇O2
=
K = (↓)![]() T × (↑)C(a −
T × (↑)C(a −
![]() )O2
.
)O2
.
The C(a - ![]() )O2
difference increases because a decrease in
)O2
difference increases because a decrease in ![]() T
causes a much larger and primary decrease in Cv̄O2
versus a smaller and secondary decrease in CaO2
:
T
causes a much larger and primary decrease in Cv̄O2
versus a smaller and secondary decrease in CaO2
:
(↑)C(a − ![]() )O2
= C(↓ a − ↓ ↓
)O2
= C(↓ a − ↓ ↓ ![]() )O2
[103]
)O2
[103]
Thus, Cv̄O2
(and
Pv̄O2
) are much more sensitive indicators
of ![]() T because they change more with changes
in
T because they change more with changes
in ![]() T than CaO2
(or PaO2
) does (see Fig
17-27
and Fig. 17-37
).
T than CaO2
(or PaO2
) does (see Fig
17-27
and Fig. 17-37
).
The amount of CO2
circulating in the body is a function
of both CO2
elimination and production. Elimination of CO2
depends on pulmonary blood flow and alveolar ventilation. Production of CO2
(V̇CO2
) parallels O2
consumption
(V̇O2
) according to the respiratory
quotient (R):

Under normal resting conditions, R is 0.8; that is, only 80% as much CO2
is produced as O2
is consumed. However, this value changes as the nature
of the metabolic substrate changes. If only carbohydrate is used, the respiratory
quotient is 1.0. Conversely, with the sole use of fat, more O2
combines
with hydrogen to produce water, and the R value drops to 0.7.
CO2 is transported from mitochondria to the alveoli in a number of forms. In plasma, CO2 exists in physical solution, hydrated to carbonic acid (H2 CO3 ), and as bicarbonate (HCO3 - ). In the RBC, CO2 combines with Hb as carbaminohemoglobin (Hb-CO2 ). The approximate relative values of H2 CO3 (H2 O + CO2 ), HCO3 - , and Hb-CO2 to the total CO2 transported are 7%, 80%, and 13%, respectively.
In plasma, CO2
exists both in physical solution and
as H2
CO3
:
H2
O + CO2
![]() H2CO3
(17)
H2CO3
(17)
The CO2
in solution can be related to PCO2
by the use of Henry's law[106]
:
PCO2
× α
= [CO2
] in solution (18)
where α is the solubility coefficient of CO2
in plasma (0.03 mmol/L/mm
Hg at 37°C). However, the major fraction of CO2
produced passes into
the RBC. As in plasma, CO2
combines with water to produce H2
CO3
.
However, unlike the slow reaction in plasma, in which the equilibrium point lies
toward the left, the reaction in an RBC is catalyzed by the enzyme carbonic anhydrase.
This zinc-containing enzyme moves the reaction to the right at a rate 1000 times
faster than in plasma. Furthermore, nearly 99.9% of the H2
CO3
dissociates to HCO3
-
and hydrogen ions (H+
):

The H+
produced from H2
CO3
in the production of
HCO3
-
is buffered by Hb (H+
+
Hb ![]() HHb). The HCO3
-
produced passes out of
the RBC into plasma to perform its function as a buffer. To maintain electrical
neutrality within the RBC, chloride ion (Cl-
) moves in as HCO3
-
moves out (Cl-
shift). Finally, CO2
can combine with Hb in
the erythrocyte (to produce Hb-CO2
). Again, as in HCO3
-
release, an H+
ion is formed in the reaction of CO2
and Hb.
This H+
ion is also buffered by Hb.
HHb). The HCO3
-
produced passes out of
the RBC into plasma to perform its function as a buffer. To maintain electrical
neutrality within the RBC, chloride ion (Cl-
) moves in as HCO3
-
moves out (Cl-
shift). Finally, CO2
can combine with Hb in
the erythrocyte (to produce Hb-CO2
). Again, as in HCO3
-
release, an H+
ion is formed in the reaction of CO2
and Hb.
This H+
ion is also buffered by Hb.
Just as the percent saturation of Hb with O2 is related to PO2 (described by the oxy-Hb curve), so is the total CO2 in blood related to PCO2 . Additionally, Hb has variable affinity for CO2 ; it binds more avidly in the reduced state than as oxy-Hb.[98] The Bohr effect describes the effect of PCO2 and [H+ ] ions on the oxy-Hb curve. Hypercapnia and acidosis both shift the curve to the right (reducing the oxygen-binding affinity of hemoglobin), and hypocapnia and alkalosis both shift the curve to the left. Conversely, the Haldane effect describes the shift in the CO2 dissociation curve caused by oxygenation of Hb. Low PO2 shifts the CO2 dissociation curve to the left so that the blood is able to pick up more CO2 , as occurs in capillaries of rapidly metabolizing tissues). Conversely, oxygenation of Hb (as occurs in the lungs) reduces the affinity of Hb for CO2 , and the CO2 dissociation curve is shifted to the right, thereby increasing CO2 removal.
|
|
|
|
|
|
|
|
|
|
|
|
|