|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The ultrastructural appearance of an alveolar septum[107] is schematically depicted in Figure 17-29 . Capillary blood is separated from alveolar gas by a series of anatomic layers: capillary endothelium, endothelial basement membrane, interstitial space, epithelial basement membrane, and alveolar epithelium (of the type I pneumocyte).
On one side of the alveolar septum (the thick, upper [see Fig. 17-29 ], fluid- and gas-exchanging side), the epithelial and endothelial basement membranes are separated by a space of variable thickness containing connective tissue fibrils, elastic fibers, fibroblasts, and macrophages. This connective tissue is the backbone of the lung parenchyma; it forms a continuum with the connective tissue sheaths around the conducting airways and blood vessels. Thus, the pericapillary perialveolar interstitial space is continuous with the interstitial tissue space that surrounds terminal bronchioles and vessels, and both spaces constitute the connective tissue space of the lung. There are no lymphatics in the interstitial space of the alveolar septum. Instead, lymphatic capillaries first appear in the interstitial space surrounding terminal bronchioles, small arteries, and veins.[108]
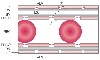 Figure 17-29
Schematic summary of the ultrastructure of a pulmonary
capillary. The upper side of the capillary has the endothelial and epithelial basement
membranes separated by an interstitial space, whereas the lower side contains only
fused endothelial and epithelial basement membranes. The dashed
arrows indicate a potential pathway for fluid to move from the intravascular
space to the interstitial space (through loose junctions in the endothelium) and
from the interstitial space to the alveolar space (through tight junctions in the
epithelium). ALV, alveolus; BM, basement membrane; ENDO, endothelium; EPI, epithelium;
IS, interstitial space; LJ, loose junction; RBC, red blood cell; TJ, tight junction.
(Redrawn from Fishman AP: Pulmonary edema: The water-exchanging function
of the lung. Circulation 46:390, 1972.)
Figure 17-29
Schematic summary of the ultrastructure of a pulmonary
capillary. The upper side of the capillary has the endothelial and epithelial basement
membranes separated by an interstitial space, whereas the lower side contains only
fused endothelial and epithelial basement membranes. The dashed
arrows indicate a potential pathway for fluid to move from the intravascular
space to the interstitial space (through loose junctions in the endothelium) and
from the interstitial space to the alveolar space (through tight junctions in the
epithelium). ALV, alveolus; BM, basement membrane; ENDO, endothelium; EPI, epithelium;
IS, interstitial space; LJ, loose junction; RBC, red blood cell; TJ, tight junction.
(Redrawn from Fishman AP: Pulmonary edema: The water-exchanging function
of the lung. Circulation 46:390, 1972.)
The opposite side of the alveolar septum (the thin, down [see Fig. 17-29 ], gas-exchanging-only side) contains only fused epithelial and endothelial basement membranes. The interstitial space is thus greatly restricted on this side because of fusion of the basement membranes. Interstitial fluid cannot separate the endothelial and epithelial cells from one another, and as a result, the space and distance barrier to fluid movement from the capillary to the alveolar compartment is reduced and composed of only the two cell linings with their associated basement membranes.[109]
Between the individual endothelial and epithelial cells are holes or junctions that provide a potential pathway for fluid to move from the intravascular space to the interstitial space and finally from the interstitial space to the alveolar space. The junctions between endothelial cells are relatively large and are therefore termed loose; the junctions between epithelial cells are relatively small and are therefore termed tight. Pulmonary capillary permeability (K) is a direct function of and essentially equivalent to the size of the holes in the endothelial and epithelial linings.
To understand how pulmonary interstitial fluid is formed, stored, and cleared, it is necessary to first develop the concepts that (1) the pulmonary interstitial space is a continuous space between the periarteriolar and peribronchial connective tissue sheath and the space between the endothelial and epithelial basement membranes in the alveolar septum and (2) the space has a progressively negative distal-to-proximal ΔP.
The concepts of a continuous connective tissue sheath-alveolar septum interstitial space and a negative interstitial space ΔP are prerequisite to understanding interstitial fluid kinetics ( Fig. 17-30 ). After entering the lung parenchyma, both the bronchi and arteries run within a connective tissue sheath that is formed by an invagination of the pleura at the hilum and ends at the level of the bronchioles ( Fig. 17-30A ). Thus, there is a potential perivascular and peribronchial space, respectively, between the arteries and the bronchi and the connective tissue sheath. The negative pressure in the pulmonary tissues surrounding the perivascular connective tissue sheath exerts a radial outward traction force on the sheath. This radial traction creates negative pressure within the sheath that is transmitted to the bronchi and arteries and tends to hold them open and increase their diameters (see Fig. 17-30 ).[109] The alveolar septum
 Figure 17-30
A, Schematic diagram of
the concept of a continuous connective tissue (CT) sheath-alveolar septum interstitial
space. The entry of the main stem bronchi and pulmonary artery into the lung parenchyma
invaginates the pleura at the hilum and forms a surrounding connective tissue sheath.
The connective tissue sheath ends at the level of the bronchioles. The space between
the pulmonary arteries and bronchi and the interstitial space is continuous with
the alveolar septum interstitial space. The alveolar septum interstitial space is
contained within the endothelial and epithelial basement membranes of the capillaries
and alveoli, respectively. B, Schematic diagram showing
how interstitial fluid moves from the alveolar septum interstitial space (no lymphatics)
to the connective tissue interstitial space (lymphatic capillaries first appear).
The mechanisms are a negative-pressure gradient (sump), the presence of one-way
valves in the lymphatics, and the massaging action of arterial pulsations. (Redrawn
with modification from Benumof JL: Anesthesia for Thoracic Surgery, 2nd ed. Philadelphia,
WB Saunders, 1995, Chapter 8.)
Figure 17-30
A, Schematic diagram of
the concept of a continuous connective tissue (CT) sheath-alveolar septum interstitial
space. The entry of the main stem bronchi and pulmonary artery into the lung parenchyma
invaginates the pleura at the hilum and forms a surrounding connective tissue sheath.
The connective tissue sheath ends at the level of the bronchioles. The space between
the pulmonary arteries and bronchi and the interstitial space is continuous with
the alveolar septum interstitial space. The alveolar septum interstitial space is
contained within the endothelial and epithelial basement membranes of the capillaries
and alveoli, respectively. B, Schematic diagram showing
how interstitial fluid moves from the alveolar septum interstitial space (no lymphatics)
to the connective tissue interstitial space (lymphatic capillaries first appear).
The mechanisms are a negative-pressure gradient (sump), the presence of one-way
valves in the lymphatics, and the massaging action of arterial pulsations. (Redrawn
with modification from Benumof JL: Anesthesia for Thoracic Surgery, 2nd ed. Philadelphia,
WB Saunders, 1995, Chapter 8.)
The forces governing net transcapillary-interstitial space fluid
movement are as follows. The net transcapillary flow of fluid (F) out of pulmonary
capillaries (across the endothelium and into the interstitial space) is equal to
the difference between pulmonary capillary hydrostatic pressure (Pinside
)
and interstitial fluid hydrostatic pressure (Poutside
) and the difference
between capillary colloid oncotic pressure (πinside
) and interstitial
colloid oncotic pressure (πoutside
). These four forces produce a steady-state
fluid flow (F) during a constant capillary permeability (K) as predicted by the Starling
equation:
F = K[(Pinside
− Poutside
)
− (πinside
− πoutside
)] (20)
K is a capillary filtration coefficient expressed in mL/min/mm Hg/100 g. The filtration
coefficient is the product of the effective capillary surface area in a given mass
of tissue and the permeability per unit surface area of the capillary wall to filter
the fluid. Under normal circumstances and at a vertical height in the lung that
is at the junction of zones 2 and 3, intravascular colloid oncotic pressure (≅26
mm Hg) acts to keep water in the capillary lumen, and working against this force,
pulmonary capillary hydrostatic pressure (≅10 mm Hg) acts to force water across
the loose endothelial junctions into the interstitial space. If these were the only
operative forces, the interstitial space and, consequently, the alveolar surfaces
would be constantly dry, and there would be no lymph flow. In fact, alveolar surfaces
are moist, and lymphatic flow from the interstitial compartment is constant (≅500
mL/day). This can be explained in part by πoutside
(≅8 mm Hg) and
in part by the negative Poutside
(-8 mm Hg). Negative (subatmospheric)
interstitial space pressure would promote, by suction, a slow loss of fluid across
the endothelial holes.[111]
Indeed, extremely negative
pleural (and perivascular hydrostatic) pressure, such as may occur in a vigorously,
spontaneously breathing patient with an obstructed airway, can cause pulmonary interstitial
edema ( Table 17-5
).[112]
Relative to the vertical level of the junction of zones 2 and 3, as lung height
decreases (lung dependency), absolute Pinside
increases, and fluid has
a propensity to transudate; as lung height increases (lung nondependency), absolute
Pinside
decreases, and fluid has a propensity to be reabsorbed. However,
fluid transudation induced by an increase in Pinside
is limited by a concomitant
dilution of proteins in the interstitial space and therefore a decrease in πoutside
.
[113]
Any change in the size of the endothelial
junctions, even if the foregoing four forces remain constant, changes the magnitude
and perhaps even the direction of fluid movement; increased size of endothelial junctions
(increased permeability) promotes transudation, whereas decreased size of
| Vigorous spontaneous ventilation against an obstructed airway |
| Laryngospasm |
| Infection, inflammation, edema |
| Upper airway mass (tumor, hematoma, abscess, foreign body, etc.) |
| Vocal cord paralysis |
| Strangulation |
| Rapid re-expansion of lung |
| Vigorous pleural suctioning (thoracentesis, chest tube) |
No lymphatics are present in the interstitial space of the alveolar septum. The lymphatic circulation starts as blind-ended lymphatic capillaries, first appearing in the interstitial space sheath surrounding terminal bronchioles and small arteries, and ends at the subclavian veins. Interstitial fluid is normally removed from the alveolar interstitial space into the lymphatics by a sump (pressure gradient) mechanism, which is caused by the presence of more negative pressure surrounding the larger arteries and bronchi.[114] [115] The sump mechanism is aided by the presence of valves in the lymph vessels. In addition, because the lymphatics run in the same sheath as the pulmonary arteries, they are exposed to the massaging action of arterial pulsations. The differential negative pressure, the lymphatic valves, and the arterial pulsations all help propel the lymph proximally toward the hilum through the lymph nodes (pulmonary to bronchopulmonary to tracheobronchial to paratracheal to scalene and cervical nodes) to the central venous circulation depot ( Fig. 17-30B ). An increase in central venous pressure, which is the backpressure for lymph to flow out of the lung, would decrease lung lymph flow and perhaps promote pulmonary interstitial edema.
If the rate of entry of fluid into the pulmonary interstitial space exceeds the capability of the pulmonary interstitial space to clear the fluid, the pulmonary interstitial space will fill with fluid; the fluid, now under an increased and positive driving force (PISF), will cross the relatively impermeable epithelial wall holes and the alveolar space will fill. Intra-alveolar edema fluid will additionally cause alveolar collapse and atelectasis, thereby promoting further accumulation of fluid.
|
|
|
|
|
|
|
|
|
|
|
|
|