|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Automated drug delivery implies that electronic or mechanical instrumentation performs dose rate adjustments independent of human intervention (in contrast to the manual devices described earlier). The desired target (e.g., drug concentration and clinical response) is still chosen by the clinician. Preprogrammed dosing (either bolus or infusion) is the simplest form of automated drug delivery and consists of a preprogrammed infusion (usually calculated to provide a single target blood or effect concentration) that is automatically implemented by the microprocessor in the pump.[101] Preprogrammed devices are limited in their application for intravenous anesthetic drugs because of their inability to allow the user to vary the target concentration.[102] Generally, two methods may be applied for automated target anesthetic drug delivery: model-based (a form of open-loop control) and closed-loop systems.
Both model-based and closed-loop systems require a set point ( Fig. 12-22 ).[103] The set point may best be defined as the quantifiable end point (e.g., plasma concentration, percent T1 of the electromyogram, or bispectral index value) related to the clinical objective (e.g., level of anesthesia, neuromuscular blockade). Accordingly, the set point is the value (i.e., target) that the automated system is attempting to maintain. The feedback signal is the measured (e.g., percent T1 of the electromyogram) or predicted (e.g., predicted effect-site concentration from pharmacokinetic simulation) value that has resulted from the automated delivery process.
A closed-loop system is the ideal means of automated drug delivery. Model-based drug delivery, however, provides an important alternative in circumstances in which the feedback signal cannot be measured and an appropriate model is available. A mathematical (e.g., pharmacokinetic) relationship that can simulate the physiology provides the model. Consequently, the accuracy of any model-based control system is dependent on how well the model represents the process under control. The control signal (which for automated drug delivery is the dosing scheme) is the difference between the set point and the model prediction. The control signal directs the actuator (i.e., the infusion pump) to produce the intervention necessary to obtain the set point (i.e., deliver the dosage regimen prescribed by the algorithm to the patient). This intervention results in a new feedback control signal, and the process repeats itself to maintain the patient at the target.
In an adaptive control system, the algorithm is adapted to the individual's unique response to the intervention produced by the actuator. For example, in a closed-loop system to control blood pressure, the algorithm may prescribe a 1-µg/kg/min increase in the infusion of sodium nitroprusside when the blood pressure exceeds 5 mm Hg above the target. Although this prescription may generally be appropriate, a change of this magnitude may cause a much greater drop in blood pressure than desired in a sensitive individual. After experience with the patient, an adaptive controller learns the individual's sensitivity and, by using this sensitivity, changes the
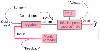 Figure 12-22
Components of a typical automated drug delivery system.
Figure 12-22
Components of a typical automated drug delivery system.
In 1968, Kruger-Thiemer[104] described the infusion regimen theoretically required to quickly achieve and maintain a constant plasma concentration of an intravenously administered drug whose kinetics are described by a two-compartment model. This regimen has become known as the "BET scheme" (see Fig. 12-4 ) and consists of an initial bolus of CT V1 , an infusion at a rate of CT ClS to replace drug eliminated from the body, and an exponentially decreasing infusion at a rate given by Equation 14 to replace drug transferred to the peripheral tissues. The section on designing dosage regimens demonstrates that the bolus portion, calculated as CT V1 , will probably be inadequate for reaching the desired effect-site concentration. Mathematically, the "ET" portion of the BET scheme is identical to the exponentially declining maintenance infusion rate shown in the section on designing dosing regimens. Precise implementation of this complex dosage regimen requires infusion rates that change continuously as a function of time until a steady state is achieved.
More than a decade after publication of Kruger-Thiemer's classic paper, Schwilden and colleagues[2] [105] [106] interfaced a microcomputer to an infusion pump and demonstrated clinical application of the BET infusion scheme. Many other groups have since implemented either the BET algorithm or modifications of this algorithm on microcomputers connected to infusion pumps. These algorithms are based on the same polyexponential equations or compartment models described previously, and they calculate the infusion rates theoretically required to obtain the desired plasma or effect-site drug concentration. When implementing these algorithms, it is necessary to consider the physical limitations of the system. For example, infusion rates must be positive or zero. Additionally, some pumps have limitations on precision and accuracy that can reduce the accuracy of the infusion.
Despite minor differences in the approaches taken by different investigators using pharmacokinetic model-driven infusion systems, all are conceptually similar. Each consists of a microcomputer interfaced to an infusion pump, as seen in Figure 12-23 . The microcomputer executes a program that incorporates the pharmacokinetic model. When using the device, the anesthesiologist enters a target plasma or effect-site drug concentration ( Fig. 12-24 ). This target concentration is based on knowledge of the pharmacokinetic-pharmacodynamic relationship of the drug and the desired effect, as well as on the individual responses of the patient. At frequent intervals (e.g., every 9 to 15 seconds), the program compares the target concentration with the current prediction of the plasma or effect-site drug concentration, which is computed by real-time simulation of a pharmacokinetic model of the drug being infused. The computer calculates the infusion rate required to achieve the desired target concentration and transmits this rate to the pump after adjusting the rate to reflect the physical capabilities of the pump. The pump then delivers drug to the patient at the desired rate.
 Figure 12-23
The CACI system used at Duke University Medical Center.
CACI consists of a laptop computer electronically linked to an infusion pump. The
desired drug plasma or effect-site concentration is entered via the keyboard.
Figure 12-23
The CACI system used at Duke University Medical Center.
CACI consists of a laptop computer electronically linked to an infusion pump. The
desired drug plasma or effect-site concentration is entered via the keyboard.
At each step, the computer makes sure that the pump has delivered the drug that it was instructed to give and checks for errors reported by the infusion pump (e.g., air in line, out of drug). The computer then calculates what the pump did during the previous interval and updates the internal pharmacokinetic model on the basis of the reported drug delivered. The cycle is completed when the computer calculates the infusion rate required over the next interval to reach the desired target concentration and transmits this rate to the infusion pump. Concurrently, the computer supplies the anesthesiologist with information about the state of the model, the state of the pump, any problems reported by the pump, the anticipated time course for elimination of drug from the patient, the total amount of drug delivered, the current infusion rate, and other information that may be of assistance in providing clinical care.
As a result of the increasing popularity of intravenous anesthesia and continuous-infusion techniques, the inherent reasonableness of pharmacokinetically based drug delivery, and the promising results achieved with automated administration of a variety of drugs by research groups around the world, pharmacokinetic model-driven infusion of propofol has become widely available worldwide, except in North America. This system consists of a commercial pump and the "Diprifusor" software, which provides the control algorithm. Reasons for the lack of availability of these devices in North America are complex but are primarily related to hostility toward computer-controlled drug infusion within the Food and Drug Administration.[107]
Several European device companies are modifying existing infusion pumps, which already contain powerful microprocessors, to provide pharmacokinetic model-driven infusions as a software-selectable option. Pharmacokinetic parameters for various drugs are already programmed into the device, and the devices have the ability to target the concentration at the site of drug effect or in plasma. Such devices are well adapted to the need for rapid titration in anesthesia. The user selects the
 Figure 12-24
Schematic illustration of pharmacokinetic model-driven
drug delivery. The physician enters the target plasma or biophase drug concentration
(Cpd
). An infusion device control algorithm uses a pharmacokinetic model
for the drug being infused to determine what the infusion rate should be for the
next infusion interval (e.g., 9 to 15 seconds). The infusion device delivers drug
to the patient, and the infusion rate is fed into a simulation of the pharmacokinetic
model to compute the current predicted plasma drug concentration (Cpp
).
The variables computed in the simulation are available to the infusion algorithm,
which then calculates the infusion rate necessary for the next 9 to 15 seconds to
achieve the target concentration. On the basis of monitored and anticipated patient
response, knowledge of approximate therapeutic plasma drug concentrations (e.g.,
Cp50
), and Cpp
, the physician can titrate Cpd
as
necessary.
Figure 12-24
Schematic illustration of pharmacokinetic model-driven
drug delivery. The physician enters the target plasma or biophase drug concentration
(Cpd
). An infusion device control algorithm uses a pharmacokinetic model
for the drug being infused to determine what the infusion rate should be for the
next infusion interval (e.g., 9 to 15 seconds). The infusion device delivers drug
to the patient, and the infusion rate is fed into a simulation of the pharmacokinetic
model to compute the current predicted plasma drug concentration (Cpp
).
The variables computed in the simulation are available to the infusion algorithm,
which then calculates the infusion rate necessary for the next 9 to 15 seconds to
achieve the target concentration. On the basis of monitored and anticipated patient
response, knowledge of approximate therapeutic plasma drug concentrations (e.g.,
Cp50
), and Cpp
, the physician can titrate Cpd
as
necessary.
Pharmacokinetic models may be built into a future generation of anesthesia workstations. These workstations could then control many different brands of infusion pumps and could provide a common platform for implementing precise titration of intravenous anesthetics in the concentration domain.
Acceptance of target-controlled drug delivery of intravenous anesthetics requires evaluation of both accuracy, defined as the difference between the predicted and the measured concentrations, and outcome of patients in whom automated drug delivery has been used. Sources of inaccuracy with pharmacokinetic model-driven devices
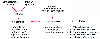 Figure 12-25
Major sources of potential error in pharmacokinetic model-driven
drug delivery. In a commercial device, the computer functions would be incorporated
into the infusion device itself.
Figure 12-25
Major sources of potential error in pharmacokinetic model-driven
drug delivery. In a commercial device, the computer functions would be incorporated
into the infusion device itself.
Inaccuracy in the software results from incorrect mathematical implementation of the pharmacokinetic model. Computer simulations can be used to test the infusion rates calculated by a software program, and thus software errors are fairly simple to identify and correct.[108] Drug delivery from the infusion pump is fairly accurate with present syringe-pump technology and thus contributes little to the overall inaccuracy of these devices. The major cause of inaccuracy is biologic variability, which may be due to two sources: (1) the pharmacokinetic model is always wrong, and (2) the patient's pharmacokinetic parameters are never those programmed into the model. The pharmacokinetic model is always wrong because individuals are far more complex than implied by simple compartmental models, and thus no such model can precisely predict the concentrations, even if the pharmacokinetic parameters in the individual were known with absolute precision. However, even if the pharmacokinetic
Performance of computer-controlled drug administration must be interpreted in terms of the therapeutic expectations of the clinician. Possible goals include accurately producing a desired concentration in plasma, precisely titrating the plasma drug concentration, producing the desired drug effect, and producing the desired time course of drug effect. Over the past decade, investigators have addressed each of these goals and have refined the performance of automated drug delivery devices in light of these goals.
The ability of an automated drug delivery system to rapidly achieve and then maintain a selected target concentration is a logical measure of the performance of such a device. The difference between the measured and
 Figure 12-26
Individual plots of the target plasma fentanyl concentration
(solid line) and measured concentration (dots)
in four separate patients.
Figure 12-26
Individual plots of the target plasma fentanyl concentration
(solid line) and measured concentration (dots)
in four separate patients.
 Figure 12-27
Plot of the measured to the predicted concentration of
fentanyl administered via CACI in 24 patients. The solid line
represents the line of identity; that is, the target concentration is equal to the
measured concentration. The dashed lines represent
a bias of ±30%.
Figure 12-27
Plot of the measured to the predicted concentration of
fentanyl administered via CACI in 24 patients. The solid line
represents the line of identity; that is, the target concentration is equal to the
measured concentration. The dashed lines represent
a bias of ±30%.
As observed earlier, it is not reasonable to expect all performance errors to be zero. However, it would be desirable if positive and negative errors offset each other so that the MDPE of an automated drug delivery device was 0%. The MDPE does not indicate the range of performance errors (because positive and negative performance errors offset each other), but it does indicate whether the plasma concentrations achieved with the device tend to overshoot the desired target (+MDPE) or undershoot the desired target (-MDPE).
Table 12-7 summarizes the accuracy of pharmacokinetic model-driven devices administering several of the intravenous anesthetic drugs.[36] [75] [101] [110] [111] [112] [113] [114] [115] [116] [117] It is clear from Table 12-7 that the expected performance of such devices, at best, tends to be around 20% to 30% MDAPE. The pharmacokinetics of propofol has probably been the most frequently tested. Coetzee and colleagues[116] tested the accuracy of three parameter sets and found those of Marsh and associates[117] and Tackley and coworkers[131] to provide the best accuracy in adult patients ( Fig. 12-28 ). To demonstrate the effect of the three different pharmacokinetic parameters on propofol dosing, the infusion rates required to produce a set of target concentrations are plotted in Figure 12-29 . The pharmacokinetic parameter set of Marsh is the one included in the Diprifusor software. Better accuracy has been obtained in children[110] because they may have less pharmacokinetic variability as a result of their general lack of chronic disease and more narrow distribution of weight at any given age (than found in adults).
Numerous pharmacokinetic parameter sets have been published for each of the drugs used in anesthesia. The pharmacokinetic parameters within each set may vary considerably, depending on a variety of factors such as the mode of drug administration (e.g., bolus versus infusion), duration of sampling, site of drug sampling (arterial versus venous), sensitivity of the assay, and evaluation of population covariates in determining the model parameters. The implications of such differences are not always obvious, and it is important to test the parameter set to determine whether the parameters provide adequate accuracy for clinical use. If the pharmacokinetic parameters are highly biased, one might expect large errors to occur in performance. Several studies in Table 12-7 show the importance of selecting the proper pharmacokinetic parameters (or at least the cost of selecting the wrong set). Shafer and colleagues[114] examined the performance of a pharmacokinetic model-driven infusion device that used the fentanyl pharmacokinetics described by McClain and Hug[122] and demonstrated an MDAPE of 61% and an MDPE of +61%. The large positive MDPE indicates that nearly all fentanyl concentrations greatly exceeded the target. The performance errors over time with the use of the McClain and Hug pharmacokinetics are shown in Figure 12-30 . Shafer and associates also examined the performance of a pharmacokinetic model-driven infusion device by use of the fentanyl pharmacokinetic parameters described by Scott and colleagues. [34] This second parameter set produced an MDAPE of 33% and an MDPE of +19%, as shown in the performance errors over time in Figure 12-30B . Although the measured concentrations tended to be higher than the target concentrations with the Scott and Stanski fentanyl parameter set, the performance was much better than that obtained with the parameter set reported by McClain and Hug. This study demonstrated that selection of the proper parameter set influenced the performance of pharmacokinetic model-driven infusion. Raemer and colleagues[110] demonstrated the same for alfentanil when they compared the performance of the alfentanil pharmacokinetic parameters reported by Maitre and coauthors[119] with the alfentanil pharmacokinetic parameters reported by Scott and coworkers.[34]
Glass and colleagues[75] examined the performance of a pharmacokinetic model-driven infusion device by use of the same fentanyl pharmacokinetics described by McClain and Hug.[122] They demonstrated an MDAPE of 21% and an MDPE of +4% (i.e., an almost completely unbiased performance). It is important to realize that the methodology in the studies by Glass and associates and Shafer and coworkers had major differences, such as arterial versus venous samples, rapid sampling after changes in target concentration versus sampling only when a pseudo-steady state was achieved, and a different set of patients—cardiac versus noncardiac. It is these differences in methodology that result in different investigators reporting differences in the performance of a pharmacokinetic model-driven infusion device using the same pharmacokinetic parameters.
With the goal of improving pharmacokinetic model-driven infusion device performance, Shafer and colleagues recalculated the optimal pharmacokinetic parameters of fentanyl directly from the observed concentrations that were obtained when fentanyl was administered through a pharmacokinetic model-driven infusion device that
| Drug | Author of Pharmacokinetic Parameter Set | Author of Study | Comments | MDAPE * (%) | MDPE * (%) |
|---|---|---|---|---|---|
| Alfentanil |
|
|
|
|
|
|
|
Schüttler and Stoeckel[118] | Ausems et al[36] |
|
|
≅22–32 |
|
|
Schüttler and Stoeckel[118] | Schüttler et al[111] |
|
≅28 | ≅0 |
|
|
Maitre et al[119] | Raemer et al[110] |
|
53 | +53 |
|
|
Scott et al[34] | Raemer et al[110] |
|
17 | +1 |
|
|
Estimated | Crankshaw et al[120] |
|
10 | +3 † |
|
|
Helmers et al[121] | Lemmens et al[112] |
|
24 | +12 |
| Fentanyl |
|
|
|
|
|
|
|
McClain and Hug[122] | Alvis et al[115] | Single target | ≅20 | ≅0 |
|
|
McClain and Hug[122] |
|
Multiple targets | ≅26 | +11 |
|
|
McClain and Hug[122] | Glass et al[123] |
|
21 | +4 |
|
|
McClain and Hug[122] | Shafer et al[114] |
|
61 | +61 |
|
|
Scott et al[34] | Shafer et al[114] |
|
33 | +19 |
|
|
Estimated |
|
|
21 ‡ | -13 ‡ |
|
|
McClain and Hug[122] | Veselis et al[113] |
|
≅40 | ≅40 |
| Sufentanil |
|
|
|
|
|
|
|
Greeley et al[124] | Kern et al[125] | Pre-CPB |
|
49 |
|
|
Greeley et al[124] | Kern et al[125] | Post-CPB |
|
32 |
| Remifentanil |
|
|
|
|
|
|
|
Minto[47] | Mertens et al[126] |
|
20 | -15 |
|
|
Egan[46] | Mertens et al[126] |
|
21 | 1 |
| Thiopental |
|
|
|
|
|
|
|
Ghoneim and Van Hamme[127] | Veselis et al[113] | Volunteers | ≅50 | ≅-50 |
| Midazolam |
|
|
|
|
|
|
|
Smith et al[128] | Kern et al[125] | Pre-CPB | 44 |
|
|
|
Smith et al[128] | Kern et al[125] | Post-CPB | 32 |
|
|
|
Greenblatt et al[129] | Veselis et al[113] | Volunteers | ≅100 | ≅100 |
| Propofol |
|
|
|
|
|
|
|
Schüttler et al[111] | Schüttler et al[111] |
|
≅22 | ≅-12 |
|
|
Dyck et al[130] | Coetzee et al[116] |
|
20 | 42 |
|
|
Tackley et al[131] | Coetzee et al[116] |
|
20 | -1 |
|
|
Marsh et al[117] | Coetzee et al[116] |
|
23 | -6 |
|
|
Marsh et al[117] | Marsh et al[117] | Pediatric patients | 25 | -18.5 |
|
|
Estimated | Marsh et al[117] | Pediatric patients | 16 § | +3 § |
|
|
Gepts et al[132] | Veselis et al[113] | Volunteers | ≅25 | 0 |
|
|
Gepts et al[132] | Glass et al[133] |
|
29 | +5 |
| CPB, cardiopulmonary bypass; MDAPE, median absolute performance error; MDPE, median performance error. | |||||
Marsh and coworkers[117] took the same approach to optimizing the performance of a pharmacokinetic model-driven infusion device administering propofol to children. They initially used a pharmacokinetic parameter
 Figure 12-28
Evaluation of three pharmacokinetic parameter sets for
propofol. Plotted is the measured-to-predicted concentration ratio for samples obtained
during administration of propofol through a target-controlled infusion device programmed
with the pharmacokinetics derived by Dyck, Marsh, or Tackley (n
= 10 per group). A ratio of 1 means that measured equals predicted. Note that the
pharmacokinetic parameters of Dyck provide a consistently positive bias.
Figure 12-28
Evaluation of three pharmacokinetic parameter sets for
propofol. Plotted is the measured-to-predicted concentration ratio for samples obtained
during administration of propofol through a target-controlled infusion device programmed
with the pharmacokinetics derived by Dyck, Marsh, or Tackley (n
= 10 per group). A ratio of 1 means that measured equals predicted. Note that the
pharmacokinetic parameters of Dyck provide a consistently positive bias.
Several other studies have also demonstrated that pharmacokinetic parameters vary in certain subsets of patients. For example, the pharmacokinetic parameters of propofol for patients presenting for open heart surgery are different from the previously verified pharmacokinetic
 Figure 12-29
Infusion rates (milliliters per hour) calculated to achieve
propofol target concentrations of 4 (10 minutes), 3 (10 minutes), 4 (20 minutes),
and 2 µg/mL (20 minutes) based on the three pharmacokinetic parameter sets
of Dyck, Marsh, and Tackley.
Figure 12-29
Infusion rates (milliliters per hour) calculated to achieve
propofol target concentrations of 4 (10 minutes), 3 (10 minutes), 4 (20 minutes),
and 2 µg/mL (20 minutes) based on the three pharmacokinetic parameter sets
of Dyck, Marsh, and Tackley.
Interest has recently focused on what other factors may be responsible for alterations in pharmacokinetic parameters, with the objective of reducing variability and increasing the accuracy of TCI systems. These factors include, among others, the influence of age, gender, hemorrhagic shock, and the administration of a second drug. Numerous studies have investigated the effect of age (both young and old) on pharmacokinetic parameters. For propofol and fentanyl, it has been shown that an adult pharmacokinetic set performs poorly in children and that age-specific parameters improve accuracy.[117] [134] Although it is well known that pharmacokinetic parameters are altered in the elderly, it has not been well established whether these changes are sufficiently different to have an impact on the accuracy of the adult parameter sets that are used in a TCI device. The effect of gender on pharmacokinetics has been variable. For propofol, Vuyk and colleagues have recently demonstrated an effect of gender on propofol pharmacokinetic parameters in the elderly.[137] In a pharmacokinetic analysis of several pooled databases of propofol, Schuttler found that gender was not a significant covariate.[138] Similarly, there is no clear indication that gender has a significant effect on opioid pharmacokinetic parameters. Several authors have looked at the effect of hemorrhagic shock on pharmacokinetic parameters. With the use of a pseudo-steady-state model of propofol, compensated hemorrhage increased propofol concentrations by only 20%; however, when uncompensated shock was induced, propofol concentrations increased by 375%.[139]
Using a non-steady-state model, Egan and associates found that hemorrhagic shock reduced central clearance and central volumes of fentanyl[140] and remifentanil[141] and resulted in higher fentanyl concentrations in animals in shock. Investigators have also focused on the impact
 Figure 12-30
Performance errors over time for fentanyl by use of the
pharmacokinetics reported by McClain and Hug (A) and
Scott and Stanski (B) and a parameter set derived
from these observations (C). (From Shafer
SL, Varvel SL, Aziz N, et al: The pharmacokinetics of fentanyl administered by computer
controlled infusion pump. Anesthesiology 73:1091–1102, 1990.)
Figure 12-30
Performance errors over time for fentanyl by use of the
pharmacokinetics reported by McClain and Hug (A) and
Scott and Stanski (B) and a parameter set derived
from these observations (C). (From Shafer
SL, Varvel SL, Aziz N, et al: The pharmacokinetics of fentanyl administered by computer
controlled infusion pump. Anesthesiology 73:1091–1102, 1990.)
Not only is there interindividual variability, but significant intraindividual variability may also occur. Hill and colleagues[147] studied volunteers to learn the individual's own pharmacokinetics for morphine and alfentanil. These investigators then used that volunteer's unique pharmacokinetic values to subsequently control a pharmacokinetic model-driven infusion device. With this technique of personalized pharmacokinetics, they
It is unlikely that we will have the opportunity to conduct a full pharmacokinetic study on patients during a single anesthetic procedure. However, Bayesian forecasting is a statistical technique by which a few measured plasma concentrations from an individual can be incorporated into pharmacokinetic parameters to improve the performance of a pharmacokinetic model-driven infusion device. Maitre and Stanski[148] demonstrated the theoretical improvement in such devices when using Bayesian forecasting for alfentanil. In this study, Maitre and Stanski demonstrated that measurement of a single plasma alfentanil concentration could potentially reduce the inaccuracy by half, to a 14% average performance error. Unfortunately, a rapid assay does not exist for any of the intravenous drugs used in anesthetic practice. However, there are rapid assays for lidocaine, and at least one pharmacokinetic model-driven infusion device (STANPUMP, developed by Shafer and Maitre at Stanford and available at http://anesthesia.stanford.edu/pkpd) performs real-time Bayesian forecasting using measured lidocaine levels for use in coronary care units.
 Figure 12-31
Simulated plasma fentanyl concentrations over time for
a brief anesthetic showing the rapid rise and fall and impression of precise titration
that can be created by a target-controlled drug delivery device (A).
B, Effect-site concentrations for the same anesthetic
course demonstrating that precise control of the plasma concentration does not necessarily
translate into precise control at the site of drug effect. (Adapted from
Shafer SL, Gregg K: Algorithms to rapidly achieve and maintain stable drug concentrations
at the site of drug effect with a computer controlled infusion pump. J Pharmacokinet
Biopharm 20:147–169, 1992.)
Figure 12-31
Simulated plasma fentanyl concentrations over time for
a brief anesthetic showing the rapid rise and fall and impression of precise titration
that can be created by a target-controlled drug delivery device (A).
B, Effect-site concentrations for the same anesthetic
course demonstrating that precise control of the plasma concentration does not necessarily
translate into precise control at the site of drug effect. (Adapted from
Shafer SL, Gregg K: Algorithms to rapidly achieve and maintain stable drug concentrations
at the site of drug effect with a computer controlled infusion pump. J Pharmacokinet
Biopharm 20:147–169, 1992.)
In the absence of rapid assays for anesthetic drugs, the obvious method to decrease variability is to observe the drug effect and adjust the target to the concentration that produces the desired level of drug effect.[30] [31] As discussed under "Pharmacodynamic Considerations," by knowing the rate constant ke0 , it is possible to model the concentration at the site of drug effect. Figure 12-31 shows the plasma fentanyl concentrations that could theoretically be obtained over the course of a brief operation. Figure 12-31B shows the fentanyl concentrations at the effect site for the same anesthetic shown in Figure 12-31A . Even if the pump produced precisely the concentrations shown in Figure 12-31A (i.e., had perfect performance), the lag between concentrations in plasma and the site of drug effect would, at best, produce a very slurred response because of the equilibration delay between plasma and the effect site. Several pharmacokinetic model-driven infusion devices now simultaneously display both the plasma and the effect-site concentrations. Such devices permit the clinician to target the concentration at the effect site rather than plasma to provide more precise control of drug effect.
By targeting the effect site, an overshoot in the plasma concentration of the drug is produced. The ke0 used to model the effect site for intravenous anesthetics has been derived from surrogate measures of effect (i.e., derivatives of the EEG). Thus, it is important to establish for TCI devices targeting the effect site that the ke0 value used actually results in the desired effect. Glass and colleagues[149] and Struys and associates[150] conducted similar studies in which they targeted either a plasma or an effect-site concentration of propofol and then observed
In summary, automated drug delivery devices appear to be able to achieve and maintain desired plasma concentrations with a median expected error of about 20% to 30% of the target. Although pharmacokinetic analysis after automated drug delivery results in a reduction in the median error to closer to 20% in adults and possibly lower in children, biologic variability sets a limit on the expected accuracy of these devices. More accurate performance could be obtained with Bayesian feedback and the use of rapid intraoperative assays, but this technique is unavailable for the intravenous drugs used in anesthetic practice. However, the equilibration delay between plasma and the effect site is such that an excessive focus on plasma concentrations may be unwarranted. Instead, learning about the pharmacokinetic/pharmacodynamic characteristics of the specific patient by titrating to a readily measured drug effect appears to be the most promising method for optimizing the performance of automated drug delivery devices to the clinical needs of the individual patient.
Evaluation of outcome with TCI devices is more difficult than evaluation of accuracy because of the difficulty of precisely measuring patient outcome. Because contemporary anesthetic practice produces very little morbidity, outcome measures are usually based on readily measured variables, such as blood pressure, heart rate, and duration of recovery room stay, rather than on substantive measures of patient morbidity. Evaluation of outcome with automated drug delivery devices requires a comparison of manual methods of administration of the same drug with automated drug delivery of intravenous anesthetics and volatile anesthetics. Numerous studies have now evaluated the administration of intravenous anesthetics by automated drug delivery devices for both brief and complex surgery, in children and adults, for short-term sedation, for prolonged sedation (days), and for chronic pain management. Unfortunately, very few outcome studies comparing automated drug delivery devices and manual delivery systems have thus far been conducted.
Ausems and colleagues[22] compared pharmacokinetic model-driven administration with intermittent bolus administration of alfentanil. Automated drug delivery produced fewer episodes of muscular rigidity, hypotension, and bradycardia on induction. Automated drug delivery during maintenance resulted in significantly fewer hemodynamic response incidents and thus resulted in a greater percentage of anesthesia time within 15% of the desired blood pressure and heart rate. Recovery after TCI was associated with significantly less use of naloxone for adequate ventilation.
Pharmacokinetic model-driven infusion of fentanyl during cardiac surgery resulted in greater hemodynamic control with fewer additional drug interventions and significantly fewer episodes of either hypotension or hypertension than occurred with bolus dose administration.[115] A small study comparing manual alfentanil administration with pharmacokinetic model-driven infusion of alfentanil showed no statistical differences during maintenance or recovery.[123] Theil and coworkers[100] compared double-blind manual administration of fentanyl/midazolam with pharmacokinetic model-driven infusion of these two anesthetics in a small group of patients undergoing cardiac surgery. Both systems were titrated simultaneously (one containing placebo), with the aim of maintaining hemodynamics within 20% of baseline values. Both systems were equally effective in providing hemodynamic control as dictated by the protocol. The most significant difference between the two modes of delivery was the greater variability in drug plasma concentrations in the manual group. This finding suggested that pharmacokinetic model-driven infusion maintained patients within a narrower therapeutic range. The advantages of this mode of infusion would be most important with drugs that have a narrow therapeutic window. This small comparative study, although it did not show many differences in outcome, demonstrated that target-controlled drug delivery was at least equal to manual drug delivery. Many pertinent outcome parameters, such as recovery times, were not measured in this study. Thus, there remains a need to establish which of the theoretical patient benefits will be realized with model-based target-controlled delivery versus manual delivery of intravenous drugs.
Several comparative studies using the commercial TCI device for propofol have been performed. Struys and colleagues[153] (N = 90), Russel and associates[154] (N = 160), and Servin in a large multicenter study (N = 562)[155] found small differences when they compared manual infusion of propofol with TCI administration. Interestingly, in the TCI groups, the maintenance infusion rate tended to be higher but was associated with a lower incidence of movement by patients. Both Servin and Russel and coworkers noted a marked preference of users for the TCI system, even though this was their first use of the device. In contrast, when TCI propofol or manual propofol was titrated to a target bispectral index, no differences were observed between the two groups.[156] Manual versus TCI delivery of remifentanil has also been evaluated in patients undergoing carotid surgery. [157] TCI provided improved hemodynamic control both intraoperatively and postoperatively with less remifentanil and similar propofol infusion rates. As discussed, targeting a plasma concentration does not provide optimal control of effect.
Pharmacokinetic model-driven infusion of propofol compares favorably with thiopental/isoflurane anesthesia for general surgery lasting several hours. [159] Induction, maintenance, and recovery were similar between the two groups. More recently, Godet and colleagues compared sevoflurane and propofol followed by isoflurane with TCI propofol in patients undergoing carotid surgery. They found minor differences in hemodynamics that tended to favor TCI propofol and no differences in recovery parameters.[160] The results of this study imply that given the appropriate means of administration, intravenous anesthesia can be equal to inhaled anesthetics in providing the objectives of anesthesia, that is, rapid induction and recovery with stable maintenance. Suttner and associates examined cost and the recovery characteristics of target-controlled propofol infusion regimens versus standard methodologies.[161] They found that "target-controlled infusion/total IV anesthesia was associated with the largest intraoperative costs but allowed the most rapid recovery from anesthesia, was associated with fewest postoperative side effects, and permitted earlier discharge from the postanesthesia care unit."
In noncomparative studies, pharmacokinetic model-driven infusion has been used to administer most of the potent opioids, as well as the hypnotics. Different anesthetic techniques have also been tested with pharmacokinetic model-driven infusion devices, including nitrous-narcotic anesthesia, supplementation with volatile anesthetics, total intravenous anesthesia and sedation for monitored anesthesia care, and intensive care unit sedation. In all these studies, outcome, as measured by hemodynamics and recovery, has been within the expectations of normal clinical care. Etomidate, methohexital, midazolam, propofol, thiopental, alfentanil, fentanyl, remifentanil, and sufentanil have all been used with TCI. When these drugs were used with target-controlled drug delivery systems for total intravenous anesthesia or to supplement nitrous oxide or volatile anesthetics, hemodynamics was well maintained during induction and intubation, as well as during maintenance. Recovery milestones were reached at times comparable to those of similar drug combinations used in manual infusion schemes. In none of these numerous studies have authors commented on adverse outcome resulting from target-controlled drug delivery.
The clinical experience with TCI devices is now enormous. A MEDLINE search of "target controlled infusion" generated over 200 articles (May 31, 2003). Clinical experience with TCI devices is now in the millions. It is only in North America that TCI is not a part of routine practice, the unfortunate result of unyielding opposition toward anesthetic drug-device combinations at the U.S. Food and Drug Administration.
Target-controlled infusion devices have also been used to provide patient-controlled analgesia. Hill and colleagues investigated patient-controlled anesthesia devices delivering morphine, fentanyl, or alfentanil[147] [162] and the clinical utility of such devices. [163] [164] [165] [166] Van den Nieuwenhuyzen and associates[58] demonstrated advantages of such a system with alfentanil over routine morphine patient-controlled anesthesia.
From the body of literature presently available, it appears that automated drug delivery of intravenous anesthetics is at least equal to manual delivery of these drugs. Intravenous drug administration using target-controlled drug delivery is analogous to inhalational vapor delivery using a calibrated vaporizer. Like the vaporizer, pharmacokinetic model-driven infusion facilitates drug delivery based on plasma or biophase concentration rather than drug dosage. Variability occurs with the use of a calibrated vaporizer, including variability in the accuracy of drug delivery with vaporizers, slow equilibration between the fresh gas flow and the circuit at low flow rates, and variable uptake by the patient. This variability does not particularly complicate titration of the inhaled anesthetics. The variability with target-controlled drug delivery is of a similar magnitude, and just as with the vaporizers, the variability associated with target-controlled drug delivery does not particularly complicate titration of intravenous anesthetics. Indeed, the time-varying stresses of surgery and the variability in patient response require that the anesthesiologist titrate the administration of potent inhaled anesthetics by using the calibrated vaporizer as a tool. These same factors require that the anesthesiologist vigilantly titrate the infusion of intravenous anesthetic drugs when using automated drug delivery as a tool.
Target-controlled drug delivery has advantages beyond being a delivery system to simplify the clinical administration of intravenous anesthetics. Probably the most significant contribution of these delivery devices is their use in research. The pseudo-steady state that can be almost instantly achieved with these devices permits the plasma and effect site to rapidly come into equilibrium, which is critically important in studies examining concentration-response relationships (i.e., the C50 values) for anesthetic drugs.[22] [37] [56] In addition, these systems enable interactions involving intravenous drugs to be clearly defined. [71] [72] [73] Closed-loop target delivery will eventually enable unbiased and more precise comparisons between drugs. Finally, as noted earlier, target-controlled delivery has provided us with the ability to prospectively test pharmacokinetic parameter sets and modeling approaches and may ultimately provide the most suitable pharmacokinetic parameters for any intravenous drug delivery.[114] [117] [135] [167] Purely as a research tool, automated drug delivery has already had a major impact on anesthetic practice.
The next step in automated drug delivery is to feed the measured drug effect directly back into the automated drug delivery device and permit the model to be updated based on observed measures of drug effect, thus providing a closed-loop system.[4] [7] [15] [16] [17] [18] [19] In this instance, the set point is the measured effect rather than the drug concentration. It has been proposed that closed-loop control of anesthesia may provide several advantages: increased stability of the control variable because of more frequent sampling of the effect and more frequent adjustment of
The simplest form of a closed-loop system is an on-off controller. [169] The infusion pump is programmed to give only one infusion rate. If the effect of the drug reduces the measured effect, the pump will turn on when the measured effect is greater than the set point (positive error) and switch off when the error is negative. Such a system will oscillate around the targeted effect. Several modifications of this simple closed-loop system have been developed that are especially important for their implementation in complex biologic systems. The next engineering modification that has been implemented to improve the performance of closed-loop devices is adjustment of the infusion rate according to the error. A proportional controller will adjust the infusion rate in accordance with the size of the error. Depending on the rapidity with which the error changes, this adjustment may also lead to overshooting or undershooting of the target, and thus the integral of the error is used to minimize this deviation. This process leads to a reduction in error but may lead to a systemic bias. To prevent such bias, the derivative of the error is also used. Most mechanical closed-loop systems are PID controllers. Biologic systems are more complex in that there is a second- or third-order relationship between the infusion dose and the effect and, in addition, sensitivity between individuals may vary by a factor of 10-fold. To deal with these factors, two further modifications in control have been used. The first is that the change in dose, based on the error, is determined by a pharmacokinetic/pharmacodynamic model. Second, as the system learns the sensitivity of the individual, the model "adapts" to that individual. Adaptation may be achieved simply by adjusting the gain, by adjusting the relationship between concentration and effect, or by using fuzzy logic. Several investigators have developed closed-loop target control systems for blood pressure,[4] [5] [6] [7] [170] neuromuscular blockade,[171] and anesthesia.[15] [16] [17] [18] [168] [172] [173] [174] [175] [176] [177] [178] Though not yet commercially available, closed-loop systems for blood pressure and neuromuscular blockade have demonstrated an ability to provide excellent control with zero bias and an MDPE and MDAPE within 10%.
As mentioned earlier, the ability to measure the adequacy of anesthesia is limited. Rather, anesthesia is a composite of several effects: hypnosis or loss of consciousness, amnesia, analgesia, lack of movement, and hemodynamic stability. Several derivatives of the EEG now show a strong correlation with hypnosis/loss of consciousness. Fortunately, amnesia is also reflected by the EEG signal and appears to precede the derived EEG values associated with drug-induced loss of consciousness. The major component of anesthesia for which we do not have a monitor of effect is analgesia. This shortcoming continues to be an impediment to the general application of closed-loop anesthesia. Noxious stimuli during surgery are extremely variable. Intravenous hypnotics (with the exception of ketamine) have limited or no analgesic activity. Thus, a closed-loop system based on a derivative of the EEG may provide perfect hypnosis in the absence of a stimulus, but awakening will occur as soon as a noxious stimulus is administered. To overcome this hurdle, investigators have taken several approaches to test closed-loop systems for anesthesia in patients. They have been used in combination with a regional anesthetic, thereby ablating any painful stimulus reaching the brain. Others have used a low-dose constant opioid concentration for superficial surgery or high-dose opioid for more extensive surgery. Another interesting approach is a combination of an analgesic concentration of opioid and application of a noxious stimulus (e.g., tetany) at regular short intervals, thus minimizing oscillations caused by surgical stimuli.
For maintenance of anesthesia, the question remains whether closed-loop systems actually provide any advantage over either manual or TCI delivery of intravenous anesthetics. Morley and coworkers compared closed-loop administration of isoflurane or a propofol/alfentanil mixture with manual control of these agents to provide a bispectral index of 50.[179] The closed-loop system used a PID control algorithm. The authors were unable to demonstrate any advantage of their closed-loop system over manual control when intraoperative control of the bispectral index, hemodynamic stability, intraoperative drug use, or recovery parameters were used as measures of outcome.
Struys and colleagues also performed a study to determine whether a closed-loop system has any advantages over manual administration of propofol for lower abdominal surgery.[176] Their approach was to have the anesthesiologist performing manual control have clinical criteria of adequate anesthesia as the target of control, whereas for the closed-loop system, the bispectral index was used as the set point. Struys and colleagues also used a model-based adaptive control system. During induction, the system administered staggered increasing propofol concentrations so that it could construct a sigmoid Emax model, thereby "learning" the individuals' concentration-effect relationship. The sigmoid Emax model developed during induction could change based on the degree of noxious stimulation and the opioid concentration. To account for this, the investigators allowed a left or right shift of the Emax curve that corresponded with the new bispectral index value and the predicted concentration. A new target concentration was determined from this new Emax curve, and this concentration was then delivered to return the bispectral index to its target. All patients received 0.5 µg/kg/min of remifentanil. The closed-loop control group achieved statistically significantly better control of both its control variable (bispectral index of 89+, 10%) and systolic pressure (51+, 27%) than did the manual group (bispectral index of 49+, 29%; systolic pressure of 34+, 31%). In addition, recovery was also improved in the closed-loop group ( Fig. 12-32 ). This study does not definitively answer whether closed-loop delivery of anesthesia is better than any manual system, nor does it define what an optimal closed-loop control system is; however, its results are encouraging and should stimulate further work on developing closed-loop anesthetic drug delivery for day-to-day anesthesia. [180]
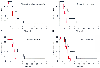 Figure 12-32
Comparison of manual versus closed-loop anesthetic control
on the time course of return of spontaneous ventilation (A),
eye opening (B), extubation (C),
and orientation (D). In each case, the more rapid
curve is the closed-loop control, and the slower curve is the manual control. (Adapted
from Struys MM, De Smet T, Versichelen LF, et al: Comparison of closed-loop controlled
administration of propofol using Bispectral Index as the controlled variable versus
"standard practice" controlled administration. Anesthesiology 95:6–17, 2001.)
Figure 12-32
Comparison of manual versus closed-loop anesthetic control
on the time course of return of spontaneous ventilation (A),
eye opening (B), extubation (C),
and orientation (D). In each case, the more rapid
curve is the closed-loop control, and the slower curve is the manual control. (Adapted
from Struys MM, De Smet T, Versichelen LF, et al: Comparison of closed-loop controlled
administration of propofol using Bispectral Index as the controlled variable versus
"standard practice" controlled administration. Anesthesiology 95:6–17, 2001.)
Closed-loop control is also an extremely valuable investigative tool. It can be used to determine the unbiased interaction between two drugs. It has been used extensively with neuromuscular blockers to determine their interaction with various anesthetics.[175] [181] [182] Similarly, closed-loop control can be used to determine the interaction between opioids and intravenous anesthetics.[183] [184] Closed-loop systems may also have the potential to diagnose different pathologic states based on drug dosing. Albrecht and colleagues observed when using closed-loop sedation with propofol that trauma patients required lower EEG set points and needed more propofol to achieve the same level of sedation. [185] As the number of investigators who have access to closed-loop systems for anesthesia increases, the utility of such devices for research is likely to increase substantially.
|
|
|
|
|
|
|
|
|
|
|
|
|