|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Volatile halogenated anesthetics have been associated with hepatotoxicity since the introduction of chloroform in 1847. Chloroform, carbon tetrachloride, and
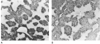 Figure 8-9
Immunochemical detection of trifluoroacetylated proteins
in the livers of mice 24 hours after treatment with (R)-
or (S)-halothane. A,
Mouse treated with (R)-halothane (original magnification
×50). B, Mouse treated with (S)-halothane
(original magnification ×50). (From Njoku D, Laster MJ, Gong DH, et
al: Biotransformation of halothane, enflurane, isoflurane and desflurane to trifluoroacetylated
liver proteins: Association between protein acylation and liver injury. Anesth
Analg 84:173–178, 1997.)
Figure 8-9
Immunochemical detection of trifluoroacetylated proteins
in the livers of mice 24 hours after treatment with (R)-
or (S)-halothane. A,
Mouse treated with (R)-halothane (original magnification
×50). B, Mouse treated with (S)-halothane
(original magnification ×50). (From Njoku D, Laster MJ, Gong DH, et
al: Biotransformation of halothane, enflurane, isoflurane and desflurane to trifluoroacetylated
liver proteins: Association between protein acylation and liver injury. Anesth
Analg 84:173–178, 1997.)
Drug-induced liver injury may be caused by intrinsic or idiosyncratic mechanisms.[120] Liver injury that is intrinsic to a particular pharmacologic agent is predictable and largely independent of host influences. Acetaminophen and chloroform are examples of pharmacologic agents that cause an intrinsic form of liver injury. The dose threshold for intrinsic toxicities may be achieved because of increased production, altered tissue sequestration, or decreased elimination of toxic metabolites. Moreover, other pharmacologic agents or chemicals, altered physiologic states, or pathologic states may affect the dose threshold for these toxicities. Nonetheless, when the dose threshold is surpassed, tissue injury may result from the direct actions of the metabolite, which may result in inhibition or modification of the enzymatic and structural systems necessary for maintaining cellular integrity.
In contrast to intrinsic mechanisms of toxicity, most pharmacologic agents produce adverse reactions through an idiosyncratic pathway. These reactions are generally host dependent, relatively uncommon, and difficult to produce in animals in which the mechanisms can be systematically studied. Nevertheless, studies done by several laboratories have suggested that many idiosyncratic reactions, including liver injury caused by inhaled anesthetics, may have an allergic or hypersensitivity basis initiated not by the parent drug itself, but instead by drug-protein adducts because small molecules are not immunogenic. These adducts are usually formed from interaction of a reactive metabolite of a drug with tissue
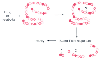 Figure 8-10
Reactive haptens can covalently bind to a native protein,
forming a hapten-protein conjugate. This conjugate can initiate an immune response
against the hapten, the native protein, or new antigenic determinants on the carrier
molecule induced by the hapten's presence.
Figure 8-10
Reactive haptens can covalently bind to a native protein,
forming a hapten-protein conjugate. This conjugate can initiate an immune response
against the hapten, the native protein, or new antigenic determinants on the carrier
molecule induced by the hapten's presence.
Two distinct types of hepatic injury have been associated with
the clinical use of halothane ( Table
8-2
). A mild injury occurs in 20% of adults who receive halothane, and
laboratory tests show mild elevations of alanine aminotransferase (ALT) and aspartate
aminotransferase (AST). The second type of injury is the fulminant form, commonly
known as halothane hepatitis, which is characterized
by elevated levels of ALT, AST, bilirubin, and alkaline phosphatase; massive hepatic
necrosis; and a fatality rate between 50% and 75%. Because of the potential for
halothane-induced hepatitis, halothane is generally not recommended for use in adults.
In several medical malpractice cases, the use of halothane resulting in injury or
| Mild Form | Fulminant Form |
|---|---|
| Incidence 1:5 | Incidence 1:10,000 |
| Repeat exposure not necessary | Multiple exposures |
| Mild elevation of ALT, AST | Marked elevation of ALT, AST, bilirubin, alkaline phosphatase |
| Focal necrosis | Massive hepatic necrosis |
| Self-limited | Mortality rate: 50% |
|
|
Antibodies to halothane-altered protein antigens |
| ALT, alanine aminotransferase; AST, aspartate aminotransferase. | |
Clinical evidence and immunochemical data have suggested that halothane hepatitis may have in some cases an allergic or hypersensitivity basis. Patients often have a history of multiple anesthetic exposures to halothane and one or more symptoms suggestive of on-going immune processes, including fever, rash, arthralgia, and eosinophilia. The first immunochemical evidence suggesting that halothane hepatitis may have an immunemediated basis was described in 1980, when it was reported that 7 of 11 patients diagnosed with halothane hepatitis had serum antibodies that reacted with the surface of hepatocytes isolated from halothane-exposed rabbits.[121] It was subsequently found that patients with halothane hepatitis often had serum antibodies that recognized native liver microsomal proteins or liver microsomal proteins that were TFA-modified by the TFA-Cl metabolite of halothane[122] [123] [124] [125] [126] [127] [128] [129] [130] (see Fig. 8-10 ). The TFA-protein adducts ( Table 8-3 ), produced by the oxidative metabolism of halothane, are thought to induce humoral or T-cell sensitization, or both, in susceptible individuals that on subsequent exposures to halothane can cause immunopathology[131] [132] ( Fig. 8-11 ). Several attempts have been made to develop an animal model of halothane hepatitis that has an immunopathologic basis, but no one has successfully accomplished this task.[133] [134] [135] [136] [137] [138] [139] [140] However, studies in guinea pigs have provided important clues regarding the mechanism of halothane hepatitis.
The guinea pig is the only animal studied in which halothane can
cause hepatotoxicity without the requirements of extensive pretreatments or other
manipulations such as exposing animals to hypoxic conditions.[141]
It is thought that the guinea pig is a model of the milder, self-limiting form of
liver injury that occurs in approximately 20% of patients administered halothane.
[135]
[136]
This
form of liver injury, however, may also be a prerequisite for the subsequent development
of severe liver damage caused by immunopathologic mechanisms. The reason for this
is that injury to hepatocytes would allow adequate levels of the TFA-protein adducts
(i.e., neoantigens) to be picked up by professional antigen presenting cells, presumably
dendritic cells. These cells may subsequently have a role in activating antigen-specific
naive B- and T-cells to produce
| Molecular mass (kd) | Protein |
|---|---|
| 100 | Endoplasmin |
| 82 | GRP-78 |
| 80 | ERP-72 |
| 63 | Calreticulin |
| 59 | Carboxylesterase |
| 57 | Protein disulfide isomerase |
| 54 | Cytochrome P450 |
| 58 | Protein disulfide isomerase (isoform) |
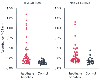 Figure 8-11
ELISA screening of halothane hepatitis patients' sera
for antibodies that react with trifluoroacetylated 58-kilodalton and native 58-kilodalton
proteins. All sera from 40 halothane hepatitis patients and 32 control patients
were assayed at a 1:100 dilution. (Adapted from Martin JL, Reed GF, Pohl
LR: Association of anti-58 kDa endoplasmic reticulum antibodies with halothane hepatitis.
Biochem Pharmacol 46:1247–1250, 1993.)
Figure 8-11
ELISA screening of halothane hepatitis patients' sera
for antibodies that react with trifluoroacetylated 58-kilodalton and native 58-kilodalton
proteins. All sera from 40 halothane hepatitis patients and 32 control patients
were assayed at a 1:100 dilution. (Adapted from Martin JL, Reed GF, Pohl
LR: Association of anti-58 kDa endoplasmic reticulum antibodies with halothane hepatitis.
Biochem Pharmacol 46:1247–1250, 1993.)
The first case of halothane hepatitis in a child was reported in 1959.[142] Since then, there have been numerous reports of hepatotoxicity and massive hepatic necrosis after halothane anesthesia in children.[143] [144] [145] [146] [147] Two retrospective studies examined the incidence of halothane-associated hepatotoxicity in children. The first study explored 165,400 halothane anesthetics in a children's hospital in the United Kingdom during the 23-year period from 1957 to 1979 and determined the incidence of halothane hepatitis in children was 1 in 82,000.[148] The second study examined 200,311 cases from 1958 to 1983 in a children's hospital in the United States. Only 1 patient in this study was concluded to have halothane hepatitis.[149] In 1987, data were reported describing halothane hepatitis in seven children between the ages of 11 months and 15 years, all of whom had received multiple halothane anesthetics.[150] The diagnosis in this study was confirmed in all but one child by the presence of serum antibodies that reacted with halothane-altered hepatocyte antigens. There was one fatality in this group, and other causes of liver diseases were excluded by the investigators. These findings indicate that the clinical syndrome of halothane hepatitis does exist in prepubertal children, although it is much less common than in adults. The reason for the difference in incidence of halothane hepatitis observed between adults and children is not clear because halothane has been found to be metabolized to a similar degree in both groups and because immune competence is known to exist from birth.[151]
The incidence of liver injury caused by fluorinated inhaled anesthetics follows the order of halothane > enflurane > isoflurane > desflurane, and it correlates with the extent of their oxidative metabolism (see Fig. 8-6 ), which is halothane (≅20%), enflurane (≅2.5%), isoflurane (≅20%), and desflurane (≅0.01%).[152] Because all of these inhaled anesthetics appear to form protein adducts that are identical or related in structure to those of halothane, it seems possible that they may cause liver injury by a mechanism similar to that of halothane, although at an appreciably lower incidence ( Fig. 8-12 ). The reduced rate results from the lower levels of potentially immunogenic protein adducts formed from these drugs compared with halothane when they are oxidatively metabolized by CYP2E1 ( Fig. 8-13 ).
Enflurane was first used in North America in 1966. Although use of enflurane is much less common today, during the period of its highest use, there were relatively few case reports of liver damage associated with it. In 1986, Eger and associates[153] reviewed 10 published reports and several unpublished reports of liver damage after enflurane administration. Many of the cases were missing critical information on the duration of anesthetic exposure, histologic confirmation of hepatic lesions, and
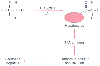 Figure 8-12
Pathway for the generation of the immune response after
anesthetic exposure in susceptible patients. Halothane is metabolized to a trifluoroacetylated
adduct that binds to liver proteins. The altered protein is seen as nonself, generating
an immune response, which on subsequent exposure leads to toxicity and cell death.
A similar process may occur after anesthetic exposure to other fluorinated drugs
that generate a trifluoroacetylated or similar adduct. TFA, trifluoroacetyl (TFA-CI).
(Adapted from Njoku D, Laster MJ, Gong DH, et al: Biotransformation of
halothane, enflurane, isoflurane and desflurane to trifluoroacetylated liver proteins:
Association between protein acylation and liver injury. Anesth Analg 84:173–178,
1997.
Figure 8-12
Pathway for the generation of the immune response after
anesthetic exposure in susceptible patients. Halothane is metabolized to a trifluoroacetylated
adduct that binds to liver proteins. The altered protein is seen as nonself, generating
an immune response, which on subsequent exposure leads to toxicity and cell death.
A similar process may occur after anesthetic exposure to other fluorinated drugs
that generate a trifluoroacetylated or similar adduct. TFA, trifluoroacetyl (TFA-CI).
(Adapted from Njoku D, Laster MJ, Gong DH, et al: Biotransformation of
halothane, enflurane, isoflurane and desflurane to trifluoroacetylated liver proteins:
Association between protein acylation and liver injury. Anesth Analg 84:173–178,
1997.
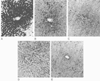 Figure 8-13
Livers from rats exposed to halothane (A),
enflurane (B), isoflurane (C),
desflurane (D), or oxygen (E),
were incubated with anti-trifluoroacetyl rabbit serum to detect the presence of trifluoroacetylated
liver proteins, after anesthetic exposure. Evidence of an immune response to trifluoroacetylated
liver proteins can be seen in A, B,
and C. No immunoreactivity is evident in D
or E.
Figure 8-13
Livers from rats exposed to halothane (A),
enflurane (B), isoflurane (C),
desflurane (D), or oxygen (E),
were incubated with anti-trifluoroacetyl rabbit serum to detect the presence of trifluoroacetylated
liver proteins, after anesthetic exposure. Evidence of an immune response to trifluoroacetylated
liver proteins can be seen in A, B,
and C. No immunoreactivity is evident in D
or E.
Isoflurane, the structural isomer of enflurane, became available clinically in 1981. The combination of its minimal oxidative metabolism and low blood-to-gas solubility contributed to the popularity of this anesthetic agent. For several years after its introduction, there were no published reports of hepatic injury after the administration of this agent. Intrigued by the paucity of cases of hepatic injury after isoflurane, Stoelting and colleagues[157] assessed the contribution of isoflurane to 45 cases of hepatic dysfunction after anesthesia that had been reported to the U.S. Food and Drug Administration. The investigators concluded that the evidence did not support an association between isoflurane and postoperative hepatic dysfunction. However, in 1991, a case report of fulminant hepatic failure after repeated isoflurane exposure was published.[158] In 1993, another case was reported in which a patient with an uneventful initial exposure to isoflurane anesthesia for cecopexy had repeated episodes of hepatitis after subsequent exposures to isoflurane.[158] In the same patient, the second isoflurane anesthetic for pyloroplasty was for a 2-hour period, and the third anesthetic for gastrojejunostomy was 30 minutes of isoflurane, followed by 150 minutes of enflurane. This patient had a far greater serum transaminase and alkaline phosphatase concentration after the second anesthetic than after the third exposure to anesthesia. Unfortunately, as with many other cases, the evidence is suggestive but insufficient to draw firm conclusions. In 2000, a case of fatal hepatotoxicity after isoflurane re-exposure was reported in which histologic evidence of centrilobular injury and microvesicular fatty changes was found.[159]
Although earlier studies of patients and volunteers do not suggest that desflurane is associated with hepatotoxicity in humans,[161] [162] there is one report of a patient who developed fulminant hepatitis 12 days after receiving a desflurane anesthetic.[119] Because this patient had been exposed to halothane 10 and 18 years previously, for periods of less than 1 hour, it is possible that the patient may have been sensitized to halothane. After exposure to desflurane, a cross-sensitization reaction may have occurred because both halothane and desflurane can form TFA-protein adducts. A second case report described a weak association between desflurane exposure and hepatotoxicity. [163] Not unexpectedly, because of the limited metabolism of desflurane, animal studies have produced little evidence for hepatotoxicity associated with desflurane.[78]
Several clinical considerations are outlined for the safe and reasonable use of fluorinated anesthetics, which have been associated with the production of acylated liver proteins and liver injury ( Table 8-4 ).
Until the year 2000, the chlorofluorocarbons (CFCs) were widely
employed as industrial refrigerants, foam-blowing agents in the manufacture of plastics,
aerosol propellants, food preservatives, and cleaning and sterilizing agents. The
major source of CFC environmental contamination was the venting of automobile and
truck air conditioning units into the atmosphere. CFCs are extremely stable, nontoxic,
and nonflammable, and they were first identified as the compounds responsible for
stratospheric ozone depletion in 1985.[164]
CFC
molecules emitted in the lower atmosphere may take up to 7 years to diffuse upward
into the stratosphere, where intense ultraviolet radiation liberates chlorine atoms
that catalyze reactions that destroy ozone molecules. Once in place, these compounds
may persist for 100 years or more. Depletion of stratospheric ozone may have adverse
health effects worldwide, such as increases in the incidence of skin
| Halothane should not be used in adult patients without a specific, well-documented indication. |
| In patients experiencing postoperative hepatotoxicity after fluorinated inhaled anesthetics, these anesthetics should be avoided in the future. |
| Despite reports of halothane hepatitis in children, halothane remains an acceptable anesthetic choice for use in children. |
| Enflurane, isoflurane, and desflurane remain safer inhaled anesthetics. |
| Anesthetic-induced hepatitis remains a diagnosis of exclusion. |
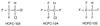 Figure 8-14
Chemical structures of the hydrochlorofluorocarbon (HCFC)
analogs of halothane: HCFC-123, HCFC-124, and HCFC-125.
Figure 8-14
Chemical structures of the hydrochlorofluorocarbon (HCFC)
analogs of halothane: HCFC-123, HCFC-124, and HCFC-125.
The addition of hydrogen atoms into CFC molecules to form hydrochlorofluorocarbon (HCFC) molecules ( Fig. 8-14 ) allows degradation of these compounds in the lower atmosphere with little effect on stratospheric ozone. HCFC-123 and -124 are used as CFC replacements. Because of the striking structural similarity between HCFCs and halothane, it seemed possible that the HCFCs would also form TFA-protein adducts similar to that of halothane ( Fig. 8-15 ) and possibly cause liver injury. In studies in rats, the relative concentrations of TFA-protein adducts formed in the liver after administration of these compounds were similar between halothane and HCFC-123, much lower for HCFC-124, and nearly undetectable for HCFC-125.[166] Acute exposure to HCFC-123 has been shown to produce severe hepatotoxicity in guinea pigs, which was enhanced by prior glutathione depletion.[167] In subchronic studies using rats and dogs, increased liver weight, slight focal liver necrosis, induction of peroxisomal activity, and hepatocellular and testicular adenomas have been found.[168] [169]
 Figure 8-15
Metabolism of halothane and the hydrochlorofluorocarbon
(HCFC) replacements, HCFC-123, HCFC-124, and HCFC-125, to an identical trifluoroacetyl
(TFA) metabolite.
Figure 8-15
Metabolism of halothane and the hydrochlorofluorocarbon
(HCFC) replacements, HCFC-123, HCFC-124, and HCFC-125, to an identical trifluoroacetyl
(TFA) metabolite.
Human liver microsomes in vitro show a much higher capacity than rat liver microsomes to bioactivate HCFC-123 to reactive metabolites, suggesting that HCFCs may be hepatotoxic to humans. HCFC-123 and HCFC-124 have been implicated in liver injury in humans.[170] In this report, nine workers were accidentally, repeatedly exposed to a mixture of HCFC-123 and HCFC-124. All nine exposed workers were affected to some degree. For one severely affected worker, liver biopsy and immunohistochemical staining for the presence of TFA-protein adducts were done. The liver biopsy sample showed hepatocellular necrosis, which was prominent in perivenular zone 3 and focally extended from portal tracts to portal tracts. TFA-adducted proteins were detected in the surviving hepatocytes. Autoantibodies against CYP2E1 or P58 were found in the sera of five of the nine affected workers. This report demonstrates that repeated exposure of human beings to HCFCs-123 and HCFC-124 can result in serious liver injury in a high proportion of the exposed population. In contrast, halothane hepatitis occurs in only a small fraction of individuals repeatedly anesthetized with this compound. A possible explanation for this difference is that halothane is administered acutely to patients, whereas the injured workers were subchronically exposed to the HCFCs. It is possible that protracted formation of TFA-adducted proteins may result in direct toxicity. Alternatively, on the basis of in vitro metabolic studies with human liver CYP2E1, exposure of human beings to HCFC-123 may result in higher concentrations of TFA-adducted liver proteins than those produced by halothane.[166] The presence of P58 and CYP2E1 autoantibodies in the serum of the exposed workers indicates that an immune component may have a role in the pathogenesis of HCFC-induced hepatotoxicity.
In several early case reports in the Japanese literature, sevoflurane was associated with postoperative hepatic dysfunction in patients ranging from 11 months to 63 years old.[171] [172] However, in all of these reports, the association between sevoflurane exposure and liver injury as manifested by elevations in levels of liver transaminases is extremely weak. In a single report from the United States,[173] postoperative liver injury was seen after sevoflurane anesthesia for appendectomy. However, this 3-year-old girl was suffering from iatrogenic acetaminophen intoxication, and her injury might have been the result of acetaminophen rather than sevoflurane. [173] A clinical study of 50 surgical patients showed no significant changes in serum transaminase levels and hepatic function after 1 to 7 MAC-hours of sevoflurane anesthesia.[174] Other studies have shown mild elevations in postoperative levels of liver transaminases after sevoflurane anesthesia.[175] [176] [177] [178] [179] Sevoflurane exposure of untreated, phenobarbital-treated, and Aroclor 1254-treated rats also showed no significant changes in the concentrations of serum transaminases, liver triglycerides, or glutathione.[180]
No discussion of immune-mediated hepatotoxicity after inhaled halogenated anesthetics is complete without considering methoxyflurane. Since its introduction into clinical practice in the United States in 1960, there have been a number of reports of hepatic dysfunction and death from hepatic coma after methoxyflurane exposure. A review of 24 cases of methoxyflurane-associated hepatitis revealed that a syndrome similar to hepatitis after halothane use may occur.[181] The researchers suggested that a rare and indirect immunologic hepatic injury might have had a direct effect on the liver by interfering with splanchnic circulation. Fortunately, in humans, the minor adverse changes in liver function appear to be reversible and may be related to dose. It is still unclear whether hepatic dysfunction, as measured by bromsulphalein retention and serum hepatic enzyme elevation, was the result of the depth and duration of the anesthetic exposure, the type of operation, the extent of preexisting hepatic disease, or methoxyflurane itself. Methoxyflurane is rarely used in current anesthesia practice.
Physiologic effects on the liver caused by the inhaled halogenated anesthetic may affect the susceptibility to hepatic dysfunction after their administration. Inhaled anesthetics can reduce hepatic blood flow to some degree, which may contribute to postoperative hepatic dysfunction. However, studies of healthy volunteers find no evidence of hypoxia or anaerobic metabolism in the liver, but hypoxia or abnormalities in hepatic synthetic function may be manifest in patients with preexisting liver damage or other illnesses. In general, surgical manipulation or disturbance of the surgical site appears to be more important in decreasing hepatic blood flow than the anesthetic agent or technique. Preexisting conditions such as chronic liver disease from alcoholism, viral infection (e.g., viral hepatitis, cytomegalovirus infection), septicemia, severe burns, nutritional deficiency, and previous or concomitant drug treatment may predipose the patient to postoperative hepatic dysfunction. Unfortunately, the patient demonstrating clinical evidence of hepatic dysfunction after exposure to inhaled halogenated anesthetics presents a challenge to the anesthesia care provider because clinical tests to assess hepatic function can be nonspecific and reflect only severe hepatic dysfunction. Even so, traditional measures of hepatic function, such as tests for serum enzymes, aminotransferases, proteins, bilirubin, and alkaline phosphatase, are still used to assess liver damage.
The reported mortality rate associated with halothane hepatitis is 40% to 75%. A variety of risk factors are commonly associated with this clinical syndrome[182] ( Table 8-5 ). Numerous studies have demonstrated that the risk of halothane hepatitis is greatly increased with use of an increasing number of anesthetics over a short period.[183] [184] Although the basis of this observation is not known, it is possible that increased exposure to TFA-proteins adducts from multiple treatments with halothane promotes the chance of a hypersensitivity reaction. Isoniazid, ethanol, and acetone can stimulate CYP2E1 levels,[185] [186] [187] [188] which may result in higher levels of protein adducts of halothane, isoflurane, and desflurane. Some evidence indicates that enzyme induction may play a role in the liver injury observed in patients with halothane hepatitis. A retrospective analysis of 279 patients
| Gender |
| Age |
| Obesity |
| Enzyme induction |
| Prior anesthetic exposure |
| Genetics |
Halothane hepatitis may have a hereditary basis in some cases, because this toxic effect has been reported in a mother and daughter, two sisters, and first cousins.[196] Lymphocytes from halothane hepatitis patients and some of their relatives are more susceptible to damage by phenytoin electrophilic intermediates than are lymphocytes from healthy controls. [197] The low incidence of the disease and the decreasing use of halothane make the study of the genetic effects difficult and emphasize the importance of developing an animal model of this disease. Several other associations exist for the development of this disease. There is a clear sex difference observed in halothane hepatitis patients. Approximately twice as many females as males develop the disease.[183] [198] [199] [200] The reason for this disparity is unclear. Most cases of halothane hepatitis have occurred in middle-aged adults, with relatively few cases reported in prepubertal children, [148] [149] and the disease is more common in obese than in nonobese patients.[201] There is no evidence to suggest that the risk of halothane hepatitis is increased in patients with liver disease unrelated to prior halothane exposure. However, because halothane can cause direct hepatotoxicity and other alternatives exist for the provision of general anesthesia, it is prudent to avoid halothane and the other fluorocarbon inhaled anesthetics in patients with preexisting hepatic dysfunction.
The diagnosis of halothane hepatitis has always been one of exclusion, in which other potential causes of liver injury such as hepatitis A, hepatitis B, hepatitis C, cytomegalovirus, Epstein-Barr virus, hepatotoxic drugs, hypotension, and hypoxia are systematically excluded as precipitating causes.[124] One of the important goals of halothane hepatitis research is the development of an assay capable of detecting sensitized patients who may be at risk for developing a hypersensitivity response on re-exposure to halothane and detecting patients with the disease ( Table 8-6 ). The assays measure serum antibodies in halothane hepatitis patients, and two general types of immunochemical assays are used. The first method is immunoblotting.[124] [202] In this procedure, test antigens are microsomal proteins from halothane-treated rats or rabbits that have been separated into constituent polypeptides by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to the surface of nitrocellulose membranes. Using this technique, 42 (62%) of 68 patients with a clinical diagnosis of halothane hepatitis have been found to test positive for the halothane-induced antibodies. [124] Although this approach provides important information about the apparent molecular mass of the neoantigens reacting with the patients' antibodies, it is laborious and time consuming. It also has the potential disadvantage of being inherently less sensitive than other methods because it involves the protein-denaturing conditions of SDS-PAGE. This could lead to a decreased level of response if a patient's antibodies were directed against, at least in part, conformational epitopes of the TFA neoantigens.
The second immunochemical assay that has been employed for the
detection of antibodies in the sera of patients with a clinical diagnosis of halothane
hepatitis is based on the more rapid, facile, and potentially more sensitive enzyme-linked
immunosorbent assay (ELISA) methodology, in which test antigen is applied directly
to the wells of a microtiter plate. One reported ELISA procedure uses microsomes
from halothane-treated rabbits as test antigen. Employing this approach, investigators
have demonstrated the presence of the antibodies in the sera of 16 (67%) of 24 patients
[203]
and 28 (72%) of 39 patients[203]
with a clinical diagnosis of halothane hepatitis. In another ELISA that employs
the TFA hapten as test antigen in the form of TFA-rabbit serum, albumin-positive
responses from patients with a clinical diagnosis of halothane hepatitis ranged from
2 (33%) of 6 patients[204]
to 5 (83%)
| History and physical examination |
| Medications |
| Prior anesthetic exposure |
| Intraoperative course |
| Postoperative course |
| Clinical tests |
| Antibody screening |
|
|
|
|
|
|
|
|
|
|
|
|
|