|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Evidence suggests that inhaled anesthetics may induce differential effects on renal physiology. For example, halothane and enflurane decrease glomerular filtration rate and renal blood flow, whereas isoflurane may also decrease glomerular filtration rate but has minimal effects on renal blood flow. The newest inhaled halogenated anesthetics have minimal effects on renal physiology. Despite these physiologic effects, renal autoregulation protects the kidney from decreases in blood flow that may be caused by the inhaled anesthetic. It has been suggested that decreases in the glomerular filtration rate caused by decreases in arterial pressure return to baseline values when arterial pressure normalizes.[207] These physiologic responses to inhaled halogenated anesthetics should be recognized, but they do not represent true toxic reactions and are not considered further.
The metabolism of certain inhaled halogenated anesthetics can produce inorganic fluoride that may be directly nephrotoxic. We review the potential for fluoride-associated nephrotoxicity after the administration of inhaled halogenated anesthetics.
Methoxyflurane is rarely used in clinical practice today; however, nephrotoxicity from inorganic fluoride released after metabolism of methoxyflurane is discussed as a basis for understanding the nephrotoxic potential of all current and future fluorinated anesthetics. Studies with methoxyflurane demonstrated an association with polyuric renal failure resulting from high levels of inorganic fluoride. [208] [209] [210] Patients with levels of inorganic fluoride less than 50 µmol/L had no evidence of renal injury. Levels of 50 to 80 µmol/L (2.5 to 3.0 MAC-hours of methoxyflurane) were associated with moderate injury and levels of 80 to 120 µmol/L (>5 MAC-hours of methoxyflurane) with severe injury. Several patients who had inorganic fluoride levels higher than 120 µmol/L died.
Various mechanisms have been proposed to explain renal injury from inorganic fluoride after methoxyflurane administration. One mechanism involves inorganic fluoride-induced reduction in adenyl cyclase activity and a subsequent effect on antidiuretic hormone.[211] Another mechanism involves effects on the renal countercurrent concentrating system by fluoride-induced increased renal medullary blood flow.[212] Nonetheless, the mechanism involving intrarenal metabolism of methoxyflurane and subsequent intrarenal production of fluoride ion is believed to be a significant cause of methoxyflurane's renal toxicity.[213] The mechanism of methoxyflurane-induced polyuric renal failure has been studied in an animal model. Vasopressin-resistant polyuric renal insufficiency similar to that seen in humans after prolonged methoxyflurane anesthesia can be consistently elicited in male Fischer 344 rats injected with sodium fluoride.[208] These rats are excellent models for studying inorganic fluoride-induced nephropathy because they demonstrate renal changes after fluoride administration similar to those seen in humans, including polyuria, hypernatremia, and increased serum hyperosmolality. These rats have a serum fluoride threshold for renal dysfunction similar to that of humans.
Despite the overall correlation between nephrotoxicity and peak serum fluoride concentrations, there is individual variability in the level of nephrotoxicity after methoxyflurane administration. Genetic heterogeneity, drug interactions, preexisting renal disease, and a host of other factors may account for the differences observed among patients. For example, enzyme induction is clearly important in Fischer 344 rats[214] with prolonged pretreatment and in human volunteers[215] treated with the enzyme-inducer phenobarbital before methoxyflurane administration, because these subjects show increased defluorination and nephrotoxicity. Other enzyme inducers such as phenytoin,[71] ethanol,[216] [217] and diazepam[218] also increase methoxyflurane defluorination in rats. One example of a drug interaction is the additive nephrotoxic effect seen in patients receiving both methoxyflurane and the aminoglycoside antibiotic gentamicin. [219] A similar effect is seen in Fischer 344 rats when concurrent administration of methoxyflurane and gentamicin synergistically promotes greater nephrotoxicity than either drug alone.[220]
In contrast to the renal effects of methoxyflurane, enflurane has been associated with only transient decreases in the renal concentrating ability. These effects are seen after prolonged administration of 9.6 MAC-hours of enflurane. [221] [222] Patients rarely show renal dysfunction after the shorter periods of enflurane anesthesia, [221] although postoperative serum F- concentrations are significantly higher than background concentrations. Compared with methoxyflurane, serum fluoride concentrations after enflurane anesthesia peak earlier and fall more rapidly, emphasizing the important role of lipid solubility in determining total fluoride exposure ( Fig. 8-16 ). In one study, peak serum fluoride concentrations from nine surgical patients averaged 22.2 µmol/L after enflurane exposures averaging 2.7 MAC-hours. The only controlled human study to show mild renal dysfunction after enflurane anesthesia involved 11 healthy volunteers.[223] After 9.6 MAC-hours of enflurane, maximum urinary osmolality after antidiuretic hormone administration was reduced from approximately 1050 to 800 mOsm, and the mean serum fluoride concentration was 33.6 µmol/L. The mild impairment of renal-concentrating ability was not associated with hypernatremia, serum hyperosmolality, or increased serum creatinine or urea nitrogen levels and therefore was not regarded as clinically significant.
Some clinicians have speculated that enflurane administered to patients with significant preexisting renal disease could produce additional renal dysfunction. Such fears have not been borne out in clinical or laboratory investigations. Studies of Fischer 344 rats with surgically induced chronic renal insufficiency did not find additional renal dysfunction.[224] [225] Results of a study of patients
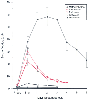 Figure 8-16
Serum inorganic fluoride (F-
) concentration
before and after administration of methoxyflurane, sevoflurane, enflurane, isoflurane,
and desflurane anesthesia. After 2 to 3 minimum alveolar concentration (MAC)-hours
of methoxyflurane, the mean peak F-
concentration was 61 ±
8 mol/L, which slowly declined. Sevoflurane anesthesia of 3.7 MAC-hours
resulted in a mean peak F-
concentration of 30.6 ± 2
mol/L, which declined more rapidly than methoxyflurane but remained elevated
for several days. After 2.7 MAC-hours of enflurane, the mean peak F-
concentration was 22.2 ± 2.8 mol/L, which also declined
over several days. There was no increase in the F-
concentration after
desflurane and almost no increase in the F-
concentration after isoflurane
administration. (Adapted from Baden JM, Rice SA: Metabolism and toxicity
of inhaled anesthetics. In Miller RD [ed]: Anesthesia,
5th ed. New York, Churchill Livingstone, 2000, p 147.)
Figure 8-16
Serum inorganic fluoride (F-
) concentration
before and after administration of methoxyflurane, sevoflurane, enflurane, isoflurane,
and desflurane anesthesia. After 2 to 3 minimum alveolar concentration (MAC)-hours
of methoxyflurane, the mean peak F-
concentration was 61 ±
8 mol/L, which slowly declined. Sevoflurane anesthesia of 3.7 MAC-hours
resulted in a mean peak F-
concentration of 30.6 ± 2
mol/L, which declined more rapidly than methoxyflurane but remained elevated
for several days. After 2.7 MAC-hours of enflurane, the mean peak F-
concentration was 22.2 ± 2.8 mol/L, which also declined
over several days. There was no increase in the F-
concentration after
desflurane and almost no increase in the F-
concentration after isoflurane
administration. (Adapted from Baden JM, Rice SA: Metabolism and toxicity
of inhaled anesthetics. In Miller RD [ed]: Anesthesia,
5th ed. New York, Churchill Livingstone, 2000, p 147.)
Several investigators have attempted to explain the differences in the nephrotoxic potentials of enflurane and methoxyflurane. One difference may be that enflurane undergoes significantly less oxidative metabolism than methoxyflurane. Moreover, enzyme induction does not significantly increase enflurane defluorination measured in vitro[69] or in surgical patients[11] receiving common enzyme-inducing drugs such as ethanol, phenobarbital, and phenytoin. In contrast, prior long-term treatment with isoniazid may result in higher than expected serum concentrations of fluoride and a transient urinary-concentrating defect after enflurane anesthesia. Work with Fischer 344 rats has shown that isoniazid significantly increases enflurane defluorination, unlike phenobarbital and phenytoin. [73] [228] A study of surgical patients has shown that approximately one half of patients treated with isoniazid on a long-term basis before enflurane anesthesia had significantly higher serum fluoride concentrations than predicted,[229] but these were not sufficiently high or sufficiently sustained to produce clinically significant renal impairment.
Isoflurane, an isomer of enflurane, is defluorinated much less than enflurane. Peak serum fluoride concentration after 6 hours of isoflurane anesthesia was only 4.4 µmol/L.[230] Isoflurane should not be associated with fluoride-associated nephrotoxicity. However, after prolonged isoflurane anesthesia for about 19 MAC-hours, peak plasma fluoride levels can be much higher after isoflurane administration than after halothane administration. [231] Forty percent of patients administered isoflurane had peak plasma fluoride levels greater than 50 µmol/L. Even so, enzyme induction does not appear to be a problem with isoflurane because phenobarbital pretreatment of rats only slightly enhances isoflurane defluorination and does not produce clinically significant increases in fluoride concentration.[69]
Because of the resistance to defluorination and the decreased likelihood of other toxicities, prolonged isoflurane administration has been investigated for uses outside the operating room. Isoflurane was investigated for its effectiveness in long-term sedation of patients on mechanical ventilation.[232] [233] In the first study, the highest serum fluoride concentration was measured for a 2-year-old child who received 73 MAC-hours of isoflurane and had a serum fluoride level of 37.4 µmol/L.[234] In the second study, pediatric patients on mechanical ventilation received isoflurane for 13 to 497 MAC-hours. The highest serum F- concentration was 26.1 µmol/L, with no alterations in serum creatinine or osmolality.[233] From these studies, we can safely conclude that isoflurane can be administered for very long periods without producing clinically significant serum fluoride concentrations; however, influences on metabolism and renal and hepatic functions should be carefully followed.
Sevoflurane is defluorinated through oxidative metabolism (see Fig. 8-7 ) to approximately the same extent as that of enflurane.[235] Clinical studies show that serum fluoride concentrations often peak above 50 µmol/L, even when sevoflurane is administered during surgery of average duration.[235] Because of sevoflurane's low blood-to-gas solubility and its rapid elimination, fluoride concentrations fall very quickly after surgery, and renal toxicities
In one study,[239] inorganic fluoride levels were compared after sevoflurane and isoflurane anesthesia. The elimination half-life of fluoride was 8 hours. The mean fluoride levels after sevoflurane were 30 µmol/L, and five of the patients had levels greater than 50 µmol/L. The mean level for isoflurane was significantly lower (3.9 µmol/L). Despite the high levels in five patients, there was no evidence of renal injury detected by blood urea nitrogen (BUN) or creatinine evaluations in this study. A similar study[234] comparing isoflurane with sevoflurane during longer (13.5) MAC-hour exposures resulted in a longer fluoride elimination half-life of about 58 hours in the sevoflurane patients and dramatically higher mean levels of inorganic fluoride (42.5 µmol/L). One half of all sevoflurane-exposed patients had fluoride levels that were higher than 50 µmol/L, but no renal injury was apparent as evidenced by BUN and creatinine measurements. Higuchi and colleagues[240] measured renal concentrating ability after 10.6 MAC-hours of sevoflurane or 8.5 MAC-hours of isoflurane in surgical patients. Mean peak fluoride levels were 41.9 ± 2.5 µM in the sevoflurane group, compared with 5.8 ± 0.4 µM in the isoflurane group.[240] There was no difference in the response to vasopressin between the groups. In a large, multinational, open-label study, patients received low-flow (1 L/min) sevoflurane (n = 98) or isoflurane (n = 90) anesthesia for at least 2 hours. BUN, creatinine, urine glucose, protein, pH, and specific gravity measurements were used to assess renal function up to 3 days after exposure. Peak fluoride levels were higher in the sevoflurane patients (40 ± 16 µM) compared with isoflurane patients (3 ± 2 µM). BUN and creatinine levels decreased in both groups, and no clinically significant differences were found with respect to any measured parameter.[241]
To accurately assess the potential for injury with sevoflurane from inorganic fluoride, more sensitive measures may be needed. These include creatinine clearance, maximal urinary osmolality (Uosmo max), urinary excretion of N-acetyl-β-glucosaminidase (NAG), β2 -microglobulin, and alanine aminopeptidase. One study[242] used several of these parameters and found evidence of transient subclinical nephrotoxicity with sevoflurane. Patients underwent peripheral orthopedic procedures lasting longer than 5 hours and were exposed to isoflurane or sevoflurane. Results were reported for two groups of sevoflurane patients, those with inorganic fluoride levels less than 50 µM/L (sevolow ) and those with levels greater than 50 µM/L (sevohigh ). This categorization was based on the levels of inorganic fluoride obtained 1 hour postoperatively: 4.8 µM/L for isoflurane, 36.8 µM/L for the sevolow group, and 55.8 µM/L for the sevohigh group. BUN, creatinine, and creatinine clearance levels were normal in all groups throughout the study. Maximal urine osmolality showed a tendency toward injury development in the sevohigh group. NAG levels were twice as high as isoflurane in the sevolow group and three times higher in the sevohigh group, with the latter group showing evidence of subclinical renal injury. Clinically, a transient loss of renal-concentrating ability occurred among one half of the patients in the sevohigh group. The dysfunction resolved within 6 days. An accompanying editorial suggested that this study raised the possibility of renal injury with sevoflurane in patients with impaired renal function and cautioned against its use in this patient group.[243] A second report of fluoride-induced renal injury in patients after sevoflurane anesthesia has been published.[244] However, a number of clinical reports have demonstrated very high levels of inorganic fluoride in some patients after sevoflurane anesthesia without obvious adverse effects. One report[245] described two such patients with refractory status asthmaticus, both treated with prolonged sevoflurane exposure under non-rebreathing conditions. Despite very high inorganic fluoride levels, no obvious injury followed. It appears that the potential for significant renal impairment or injury after sevoflurane anesthesia in healthy patients due solely to inorganic fluoride is not an important clinical problem.
Whether sevoflurane further affects renal tubular function in patients with impaired renal function is a question investigated by several groups. Cozen and coworkers[246] compared sevoflurane (n = 21) with enflurane (n = 20) at 4 L/min fresh gas flow rates in patients with chronically impaired renal function (creatinine > 1.5 mg/dL). Although peak fluoride levels were significantly higher in the sevoflurane group (25 ± 2.2 µM) than the enflurane group (13.3 ± 1.1 µM), no difference was found in postoperative renal impairment.[246] Tsukamoto and associates[247] compared sevoflurane (n = 7) with isoflurane (n = 7) in patients with moderately impaired renal function (creatinine clearance between 10 and 55 mM/min). Fluoride, urine NAG, γ-GTP, and β2 -microglobulin levels were measured up to 2 weeks after surgery. With the exception of fluoride levels (sevo group ⋙ iso group), no differences in renal parameters were observed between the groups.[247] In a later study, Morita and colleagues[248] evaluated the effect of sevoflurane (n = 15) and propofol (n = 15) anesthesia on urine concentration and aquaporin-2 (AQP2) levels. AQP2 is an arginine vasopressin-regulated water channel protein localized in the apical region of renal collecting duct cells. In both groups, plasma and urinary concentrations of arginine vasopressin increased, although plasma osmolality remained unchanged. Urinary AQP2 excretion increased in the propofol group along with changes in urinary and plasma arginine vasopressin levels. The urinary AQP2 level was significantly lower at 90 minutes in the sevoflurane group, and urine osmolality showed a transient but significant decrease in parallel with AQP2 suppression, suggesting sevoflurane might have produced transient impairment of the APQ2 response to an increase in intrinsic arginine vasopressin. However, because other inhaled anesthetics were not tested, it is not clear whether this observation applies to sevoflurane alone or is a characteristic effect of other fluorinated inhaled anesthetic agents.
Clinical studies performed with desflurane show no evidence of nephrotoxicity. Desflurane is extremely resistant to defluorination, and serum fluoride concentrations in surgical patients after exposure to desflurane are not increased above background concentrations.[249] From these findings, desflurane does not appear to be nephrotoxic.
Halothane is not significantly defluorinated under normal clinical conditions and is not nephrotoxic. In patients who received about 19 MAC-hours of halothane, peak plasma fluoride levels were much higher in the isoflurane group than in the halothane group.[237] Defluorination is enhanced slightly in rats under conditions of hypoxia and enzyme induction, although not to an extent associated with renal damage.
Fluoride-induced nephrotoxicity is a well-known entity that is historically associated with methoxyflurane administration and associated with prolonged exposure to enflurane. Surprisingly, during the administration of sevoflurane, serum inorganic fluoride levels can exceed 50 µmol/L, the level known to produce nephrotoxicity, but no correlation with sevoflurane and polyuric renal failure has been documented. Two factors may help to explain the differences seen with methoxyflurane and sevoflurane. First, it is not the peak serum fluoride concentration that determines injury, but rather the duration of the systemic fluoride increase (i.e., the area under the curve for serum fluoride). Sevoflurane is an order of magnitude less soluble than methoxyflurane and is eliminated much more rapidly from the body. Second, the liver is the primary organ of sevoflurane metabolism, whereas both the liver and kidney metabolize methoxyflurane, and it is thought that the high intrarenal fluoride production from methoxyflurane contributes to its nephrotoxicity.
|
|
|
|
|
|
|
|
|
|
|
|
|