Stereoselective Metabolism of Inhaled Anesthetics
Many drugs, including the fluorinated anesthetics, are prepared
and used therapeutically as racemic mixtures. Clinical experience has confirmed
that enantiomeric (i.e., non-superimposable mirror image) forms of drugs may differ
in their potency, pharmacologic actions, and toxicities.[89]
[90]
[91]
[92]
Such non-superimposable objects are said to be chiral.
The most common chiral origin is the presence of an asymmetric center, a carbon
atom attached to four different substitute groups. Enantiomers are optically active;
a single enantiomeric form rotates the plane of polarized light passing through an
aqueous solution of the enantiomer in a clockwise or counterclockwise direction.
The International Union of Pure and Applied Chemistry (IUPAC) preferred method for
the classification of enantiomers is the Cahn-Perlog-Ingold convention or (R/S)
classification. In this system, the four substitutions around the chiral center
are assigned priorities depending on atomic number and atomic mass. When looking
down the carbon bond to the substituent with the lowest priority, if the other substitutents
are ordered from higher to lower in a clockwise direction, the assignment is the
(R)-configuration; if counterclockwise, the (S)-configuration
is designated.
Whether the clinical use of a single stereoisomer provides significant
advantages depends on pharmacokinetic and pharmacodynamic properties of the respective
enantiomers. For example, (S)-penicillamine is used
to treat Wilson's disease, cystinuria, and rheumatoid arthritis because of toxicities
associated with the racemic drug.[93]
The calcium
channel blocker (S)-verapamil is 10 to 20 times more
potent than (R)-verapamil and appears to be a better
antiarrhythmic, antianginal, and antihypertensive agent.[94]
The cardiovascular drug (S)-propranolol is clinically
useful as an antihypertensive and antianginal agent, whereas (R)-propranolol,
which lacks cardiovascular effects, is useful in the treatment of hypothyroidism.
[95]
[96]
Ketamine
is provided for clinical use as a racemic mixture, although (S)-ketamine
has superior efficacy, faster elimination, and recovery characteristics, with fewer
side effects, ranging from restlessness and agitation to emergence reactions, compared
with racemic ketamine.[97]
(S)-Ketamine
is three times more potent than (R)-ketamine as an
anesthetic and analgesic agent, and enantiomeric stereoselectivity of hepatic metabolism
of ketamine by microsomal enzymes has been demonstrated.[98]
Ropivacaine, the (S)-enantiomer of a bupivacaine
homolog, and levobupivacaine [i.e., (S)-bupivacaine]
were developed as alternatives to racemic bupivacaine because of its known central
nervous system and cardiovascular toxicities. Ropivacaine is less potent than (S)-bupivacaine
or racemic bupivacaine in blocking sodium channels, although the anesthetic and analgesic
effects of the two drugs are similar.[99]
Perhaps
the most infamous example of enantiomeric differences between optical isomers was
observed with the drug thalidomide. The
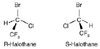 Figure 8-8
(R)- and (S)-enantiomers
of halothane.
Figure 8-8
(R)- and (S)-enantiomers
of halothane.
(R)- and (S)-isomers
of thalidomide and the racemic mixture are equally active hypnotics; however, (S)-thalidomide
is transformed into metabolites that proved to be embryogenic and teratogenic.[100]
Commercial halothane is a racemic mixture of the (R)-
and (S)-enantiomers ( Fig.
8-8
). Metabolic studies have shown that racemic halothane undergoes oxidative
metabolism catalyzed by CYP2E1 to TFA-Cl, which covalently binds to several hepatic
proteins that are believed to be involved in the immune response leading to halothane
hepatotoxicity. To investigate whether CYP2E1 catalyzes the metabolism of halothane
in a stereoselective fashion, the enantiomers of halothane were prepared and tested
in vivo for their ability to form covalently bound TFA-protein adducts in the liver
of mice. It was found that halothane underwent stereoselective metabolism, with
the (R)-isomer producing significantly greater amounts
of TFA-protein adducts in liver than the (S)-isomer
[101]
( Fig.
8-9
). Because the anesthetic potency of the isomers appears to be equal,
[102]
these findings suggest that the (S)-isomer
of halothane may be a safer inhaled anesthetic agent than the (R)-isomer
or the racemic mixture. This idea is supported by the observation that the hepatotoxicity
caused by halothane, enflurane, isoflurane, and desflurane is related to their relative
degree of oxidative metabolism in vivo to reactive acyl halide metabolites (see Fig.
8-6
). Because enflurane, isoflurane, and desflurane also contain an asymmetric
carbon atom, the potential for stereoselective metabolism exists with these agents.
Other researchers have found that the ratio of (R)-enflurane
metabolism was 1.9:1 compared with (S)-enflurane
metabolism in human liver microsomes.[60]
It has
also been demonstrated that optical isomers of isoflurane exhibit a significant difference
in anesthetic potency,[103]
differentially increase
sleep time in mice,[104]
and exhibit a modest but
significant stereoselectivity at the γ-aminobutyric acid A (GABAA
)
receptor.[105]
[106]
[107]
The clinical use of chiral anesthetic drugs
as equal mixtures of their enantiomers has been accepted practice in medicine and
anesthesia. With increased knowledge and awareness regarding drug toxicities, future
anesthetic drugs may be developed and marketed as single, more potent, and potentially
less toxic optically active isomers.
 |
 Figure 8-8
(R)- and (S)-enantiomers
of halothane.
Figure 8-8
(R)- and (S)-enantiomers
of halothane.