Use of Central Vascular Pressures to Estimate Left
Ventricular Preload
Accurate, clinically meaningful interpretation of PAC-derived
cardiac filling pressures requires a detailed understanding of the underlying physiologic
principles, particularly the relationship between left ventricular filling pressure
and preload. Although measurements such as pulmonary artery diastolic pressure and
wedge pressure are used often as measures of left ventricular filling, many factors
influence the relationship between filling
 Figure 32-46
Influence of positive-pressure mechanical ventilation
on pulmonary artery pressure. Pulmonary artery pressure should be measured at end-expiration
(#1, 15 mm Hg) to obviate the artifact caused by positive-pressure inspiration (#2,
22 mm Hg). Compare with Figure 32-27
.
(Redrawn from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill
Livingstone, 1998, Fig. 16-3.)
Figure 32-46
Influence of positive-pressure mechanical ventilation
on pulmonary artery pressure. Pulmonary artery pressure should be measured at end-expiration
(#1, 15 mm Hg) to obviate the artifact caused by positive-pressure inspiration (#2,
22 mm Hg). Compare with Figure 32-27
.
(Redrawn from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill
Livingstone, 1998, Fig. 16-3.)
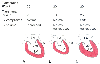 Figure 32-47
Influence of juxtacardiac pressure and ventricular compliance
on left ventricular (LV) preload. There are three interpretations of an increased
transduced pulmonary artery wedge pressure (PAWP, 20 mm Hg). A,
Juxtacardiac pressure (-5 mm Hg) and LV compliance are normal, transmural PAWP is
increased (25 mm Hg), and LV volume is increased. B,
Juxtacardiac pressure is increased (+10 mm Hg), LV compliance is normal, transmural
PAWP is decreased (10 mm Hg), and LV volume is normal or decreased. C,
Juxtacardiac pressure is normal, LV compliance is decreased, transmural PAWP is increased
(25 mm Hg), and LV volume is normal or decreased. LA, left atrium. (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 15-8.)
Figure 32-47
Influence of juxtacardiac pressure and ventricular compliance
on left ventricular (LV) preload. There are three interpretations of an increased
transduced pulmonary artery wedge pressure (PAWP, 20 mm Hg). A,
Juxtacardiac pressure (-5 mm Hg) and LV compliance are normal, transmural PAWP is
increased (25 mm Hg), and LV volume is increased. B,
Juxtacardiac pressure is increased (+10 mm Hg), LV compliance is normal, transmural
PAWP is decreased (10 mm Hg), and LV volume is normal or decreased. C,
Juxtacardiac pressure is normal, LV compliance is decreased, transmural PAWP is increased
(25 mm Hg), and LV volume is normal or decreased. LA, left atrium. (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 15-8.)
pressure and chamber volume. For example, consider how one might interpret measurement
of a PAWP of 20 mm Hg ( Fig. 32-47
).
This pressure is somewhat higher than the normal value, but depending on how this
measured wedge pressure is interpreted, different treatments would be indicated.
Proper interpretation of this filling pressure depends on two factors, juxtacardiac
pressure and ventricular compliance. The most common interpretation of a wedge pressure
of 20 mm Hg assumes that juxtacardiac pressure and ventricular compliance are normal
and leads to the diagnosis of hypervolemia, with increased left ventricular end-diastolic
volume causing the increased PAWP. One arrives at a different interpretation of
this wedge pressure if juxtacardiac pressure is increased for any reason, including
cardiac tamponade, pericardial constriction, or positive-pressure inspiration. Under
these conditions, the same elevated filling pressure may be associated with normal
or reduced left ventricular end-diastolic volume.
Finally, a wedge pressure of 20 mm Hg has a third interpretation if ventricular compliance
is decreased, such as might occur with diastolic dysfunction from myocardial ischemia,
hypertrophy, or cardiomyopathy. Under these conditions, as in the case of increased
juxtacardiac pressure, left ventricular volume may be normal or reduced despite elevated
wedge pressure (see Fig. 32-47
).
Deciding whether any given central vascular pressure is ideal
for a particular patient may be a clinical challenge. When wedge pressure is extremely
high or low, it is easy to recognize that this value must be adjusted to avoid pulmonary
edema or improve cardiac output. However, things are rarely so clear-cut in critically
ill patients, and the target value for optimal wedge pressure is uncertain and often
decided empirically. Under these circumstances, a rapid fluid challenge may be a
useful method to determine whether the PAWP value is optimal for the patient in any
particular clinical setting. An intravenous bolus of crystalloid or colloid solution
(250 to 500 mL) is given rapidly over a period of 15 minutes, and the change in wedge
pressure is measured along with other pertinent hemodynamic variables. If the baseline
wedge pressure is high or if a severe pulmonary capillary injury is present, a reduced
volume may be used. Small increases in wedge pressure after the fluid challenge
(e.g., less than 3 mm Hg) suggest that the ventricle is operating on the flat portion
of its diastolic filling curve, whereas large increases in wedge pressure (e.g.,
7 mm Hg or greater) suggest that the steep portion of the curve has been reached
and that little further increase in stroke volume and cardiac output can be achieved
without a substantial risk of producing hydrostatic pulmonary edema.[120]
[504]
As difficult as it may be to decide whether the wedge pressure
is optimal for a particular patient, even greater uncertainty confronts a physician
who uses CVP to
 Figure 32-48
Anatomic and physiologic factors that influence the relationships
between various measures of left ventricular (LV) filling and true LV preload. The
further upstream filling pressure is measured, the more confounding factors may influence
the relationship between this measurement and LV preload. CVP, central venous pressure;
LA, left atrium; LAP, left atrial pressure; LVEDP, left ventricular end-diastolic
pressure; PA, pulmonary artery; PADP, pulmonary artery diastolic pressure; PAWP,
pulmonary artery wedge pressure; P-V, pressure-volume; RA, right atrium; RV, right
ventricle. (Redrawn from Mark JB: Atlas of Cardiovascular Monitoring.
New York, Churchill Livingstone, 1998, Fig. 15-5).
Figure 32-48
Anatomic and physiologic factors that influence the relationships
between various measures of left ventricular (LV) filling and true LV preload. The
further upstream filling pressure is measured, the more confounding factors may influence
the relationship between this measurement and LV preload. CVP, central venous pressure;
LA, left atrium; LAP, left atrial pressure; LVEDP, left ventricular end-diastolic
pressure; PA, pulmonary artery; PADP, pulmonary artery diastolic pressure; PAWP,
pulmonary artery wedge pressure; P-V, pressure-volume; RA, right atrium; RV, right
ventricle. (Redrawn from Mark JB: Atlas of Cardiovascular Monitoring.
New York, Churchill Livingstone, 1998, Fig. 15-5).
determine whether cardiac preload is adequate or optimal. Clinicians have recognized
for years that in many patients, CVP may be misleading[506]
[507]
[508]
[509]
because of a number of anatomic and physiologic reasons.[294]
The diastolic pressure-volume curves for the left and right ventricles are different,
even in healthy individuals, with the left ventricle being stiffer or less compliant.
Although investigators have shown that changes in CVP, pulmonary artery diastolic
pressure, and wedge pressure all occur in the same direction,[510]
the more important question is whether small changes in CVP are clinically detectable
given that these small changes are often accompanied by much larger changes in PAWP,
simply for the reason that the right and left ventricles have different diastolic
pressure-volume relationships.[511]
The use of CVP to estimate left ventricular preload is associated
with additional interpretive problems because of the fact that the right and left
ventricles share a common septal wall and are both contained within the pericardium.
Ventricular interdependence and pericardial constraint couple changes in right and
left ventricular function such that a primary change in right ventricular filling
may produce a secondary change in left ventricular filling by altering its diastolic
pressure-volume relationship.[293]
[294]
[296]
[302]
[512]
For example, acute pulmonary artery hypertension increases right ventricular end-diastolic
volume and pressure, shifts the ventricular septum leftward, and increases left ventricular
end-diastolic pressure while simultaneously decreasing left ventricular end-diastolic
volume because of a shift in the left ventricular pressure-volume relationship to
a steeper, stiffer curve.
Finally, numerous additional anatomic, physiologic, and pathophysiologic
factors may alter the relationship between CVP and left ventricular preload ( Fig.
32-48
).[294]
The further upstream from
the left ventricle one records
the "filling pressure," the greater the number of these factors that may conspire
to alter the relationship between the monitored pressure and left ventricular preload.
In view of these considerations, it should be no surprise that reliance on CVP to
estimate left ventricular preload is often misleading in critically ill patients.
Furthermore, these same anatomic and physiologic factors may influence the relationship
between pulmonary artery diastolic pressure, wedge pressure, left atrial pressure,
and left ventricular end-diastolic pressure as measure of ventricular filling.
 Figure 32-46
Influence of positive-pressure mechanical ventilation
on pulmonary artery pressure. Pulmonary artery pressure should be measured at end-expiration
(#1, 15 mm Hg) to obviate the artifact caused by positive-pressure inspiration (#2,
22 mm Hg). Compare with Figure 32-27
.
(Redrawn from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill
Livingstone, 1998, Fig. 16-3.)
Figure 32-46
Influence of positive-pressure mechanical ventilation
on pulmonary artery pressure. Pulmonary artery pressure should be measured at end-expiration
(#1, 15 mm Hg) to obviate the artifact caused by positive-pressure inspiration (#2,
22 mm Hg). Compare with Figure 32-27
.
(Redrawn from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill
Livingstone, 1998, Fig. 16-3.)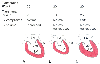 Figure 32-47
Influence of juxtacardiac pressure and ventricular compliance
on left ventricular (LV) preload. There are three interpretations of an increased
transduced pulmonary artery wedge pressure (PAWP, 20 mm Hg). A,
Juxtacardiac pressure (-5 mm Hg) and LV compliance are normal, transmural PAWP is
increased (25 mm Hg), and LV volume is increased. B,
Juxtacardiac pressure is increased (+10 mm Hg), LV compliance is normal, transmural
PAWP is decreased (10 mm Hg), and LV volume is normal or decreased. C,
Juxtacardiac pressure is normal, LV compliance is decreased, transmural PAWP is increased
(25 mm Hg), and LV volume is normal or decreased. LA, left atrium. (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 15-8.)
Figure 32-47
Influence of juxtacardiac pressure and ventricular compliance
on left ventricular (LV) preload. There are three interpretations of an increased
transduced pulmonary artery wedge pressure (PAWP, 20 mm Hg). A,
Juxtacardiac pressure (-5 mm Hg) and LV compliance are normal, transmural PAWP is
increased (25 mm Hg), and LV volume is increased. B,
Juxtacardiac pressure is increased (+10 mm Hg), LV compliance is normal, transmural
PAWP is decreased (10 mm Hg), and LV volume is normal or decreased. C,
Juxtacardiac pressure is normal, LV compliance is decreased, transmural PAWP is increased
(25 mm Hg), and LV volume is normal or decreased. LA, left atrium. (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 15-8.) Figure 32-48
Anatomic and physiologic factors that influence the relationships
between various measures of left ventricular (LV) filling and true LV preload. The
further upstream filling pressure is measured, the more confounding factors may influence
the relationship between this measurement and LV preload. CVP, central venous pressure;
LA, left atrium; LAP, left atrial pressure; LVEDP, left ventricular end-diastolic
pressure; PA, pulmonary artery; PADP, pulmonary artery diastolic pressure; PAWP,
pulmonary artery wedge pressure; P-V, pressure-volume; RA, right atrium; RV, right
ventricle. (Redrawn from Mark JB: Atlas of Cardiovascular Monitoring.
New York, Churchill Livingstone, 1998, Fig. 15-5).
Figure 32-48
Anatomic and physiologic factors that influence the relationships
between various measures of left ventricular (LV) filling and true LV preload. The
further upstream filling pressure is measured, the more confounding factors may influence
the relationship between this measurement and LV preload. CVP, central venous pressure;
LA, left atrium; LAP, left atrial pressure; LVEDP, left ventricular end-diastolic
pressure; PA, pulmonary artery; PADP, pulmonary artery diastolic pressure; PAWP,
pulmonary artery wedge pressure; P-V, pressure-volume; RA, right atrium; RV, right
ventricle. (Redrawn from Mark JB: Atlas of Cardiovascular Monitoring.
New York, Churchill Livingstone, 1998, Fig. 15-5).