|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PAC monitoring is subject to the same technical artifacts inherent in all invasive pressure monitoring techniques,
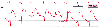 Figure 32-36
Artifactual pressure peaks and troughs in the pulmonary
artery pressure (PAP) waveform caused by catheter motion. The correct value for
pulmonary artery end-diastolic pressure is 8 mm Hg (A), although the monitor digital
display erroneously reports the PAP as 28/0 mm Hg (B). (Redrawn from Mark
JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone, 1998,
Fig. 5-6.)
Figure 32-36
Artifactual pressure peaks and troughs in the pulmonary
artery pressure (PAP) waveform caused by catheter motion. The correct value for
pulmonary artery end-diastolic pressure is 8 mm Hg (A), although the monitor digital
display erroneously reports the PAP as 28/0 mm Hg (B). (Redrawn from Mark
JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone, 1998,
Fig. 5-6.)
The most common prominent pressure artifact observed in a PAC trace is a sharp pressure spike seen immediately after the ECG R wave at onset of systole ( Fig. 32-36 ).[450] [453] At this point in the cardiac cycle, tricuspid valve closure, right ventricular contraction, and ejection set the PAC in motion and inscribe a motion artifact in the pressure waveform. Note that this pressure artifact appears at the same time as the CVP c wave and may produce a factitiously low pressure or a pressure peak. If the bedside monitor detects this artifactual pressure nadir inappropriately, it may be erroneously designated as the pulmonary artery diastolic pressure (see Fig. 32-36 ). Repositioning the PAC by advancing or withdrawing it a few centimeters often ameliorates the problem.
Another common artifact in PAC pressure measurement occurs when attempts to inflate the balloon cause the catheter tip to become obstructed and thus fail to measure intravascular pressure. This phenomenon is generally termed overwedging and is usually caused by distal catheter migration and eccentric balloon inflation that forces the catheter tip against the wall of the pulmonary artery. Rather
 Figure 32-37
Overwedging of the pulmonary artery (PA) catheter causes
artifactual waveform recordings. The first two attempts to inflate the PA catheter
balloon (first two arrows) produce a nonpulsatile,
increasing pressure caused by an occluded catheter tip. After the catheter is withdrawn
slightly, balloon inflation allows proper wedge pressure measurement (third
arrow). Before the third attempt at balloon inflation, the PA pressure
lumen is flushed. Flushing restores the appropriate pulsatile pressure detailed
to the PA and wedge pressure waveforms on the right side of the trace. (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 5-7.)
Figure 32-37
Overwedging of the pulmonary artery (PA) catheter causes
artifactual waveform recordings. The first two attempts to inflate the PA catheter
balloon (first two arrows) produce a nonpulsatile,
increasing pressure caused by an occluded catheter tip. After the catheter is withdrawn
slightly, balloon inflation allows proper wedge pressure measurement (third
arrow). Before the third attempt at balloon inflation, the PA pressure
lumen is flushed. Flushing restores the appropriate pulsatile pressure detailed
to the PA and wedge pressure waveforms on the right side of the trace. (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 5-7.)
As emphasized earlier, each PAC balloon inflation allows the catheter tip to migrate distally several centimeters to reach the wedge position. This flotation process generally occurs over several cardiac cycles. When a wedge pressure tracing appears during partial balloon inflation, it suggests that the PAC is located inappropriately in a smaller, distal branch of the pulmonary artery. The catheter should be withdrawn before overwedging results in vascular injury or pulmonary infarction. A distally positioned catheter may overwedge itself without balloon inflation. This problem must be recognized immediately by observing the waveform, and the PAC must be withdrawn until a normal PAP tracing is restored.
Pathophysiologic conditions involving the left-sided cardiac chambers or valves produce characteristic changes in the pulmonary artery and wedge pressure waveforms ( Table 32-11 ). [454] One of the most widely recognized abnormalities is the tall v wave of mitral regurgitation ( Fig. 32-38 ). Unlike a normal wedge pressure v wave produced by late systolic pulmonary venous inflow, which fills the left atrium while the mitral valve is closed, the prominent v wave of mitral regurgitation begins in
| Condition | Characteristics |
|---|---|
| Mitral regurgitation | Tall regurgitant c-v wave |
|
|
Obliteration of x descent |
| Ventricular septal defect | Tall antegrade v wave |
| Mitral stenosis | Tall a wave |
|
|
Attenuation of y descent |
| Myocardial ischemia | Tall a waves |
|
|
Tall v waves |
When large v waves are present in the wedge pressure trace, it is critically important to recognize them and be able to distinguish the wedge pressure waveform from the unwedged PAP waveform. At first glance, a wedge trace with a tall systolic v wave resembles a typical unwedged PAP trace, but closer observation reveals a number of discriminating morphologic details. The PAP upstroke is
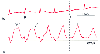 Figure 32-38
Mitral regurgitation. A tall regurgitant v wave (v)
is seen in the pulmonary artery wedge pressure (PAWP) trace and may also be noted
in the unwedged pulmonary artery pressure (PAP) waveform (arrow).
(Redrawn from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill
Livingstone, 1998, Fig. 17-5.)
Figure 32-38
Mitral regurgitation. A tall regurgitant v wave (v)
is seen in the pulmonary artery wedge pressure (PAWP) trace and may also be noted
in the unwedged pulmonary artery pressure (PAP) waveform (arrow).
(Redrawn from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill
Livingstone, 1998, Fig. 17-5.)
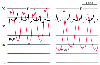 Figure 32-39
Severe mitral regurgitation. A tall systolic v wave
(v) is inscribed in the pulmonary artery wedge pressure (PAWP) trace and also distorts
the pulmonary artery pressure (PAP) trace, thus giving it a bifid appearance. The
electrocardiogram (ECG) is abnormal because of ventricular pacing. Left ventricular
end-diastolic pressure is estimated best by measuring PAWP at the time of the electrocardiographic
R wave, before onset of the regurgitant v wave. Note that mean PAWP exceeds left
ventricular end-diastolic pressure in this condition. (Redrawn from Mark
JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone, 1998,
Fig. 17-11.)
Figure 32-39
Severe mitral regurgitation. A tall systolic v wave
(v) is inscribed in the pulmonary artery wedge pressure (PAWP) trace and also distorts
the pulmonary artery pressure (PAP) trace, thus giving it a bifid appearance. The
electrocardiogram (ECG) is abnormal because of ventricular pacing. Left ventricular
end-diastolic pressure is estimated best by measuring PAWP at the time of the electrocardiographic
R wave, before onset of the regurgitant v wave. Note that mean PAWP exceeds left
ventricular end-diastolic pressure in this condition. (Redrawn from Mark
JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone, 1998,
Fig. 17-11.)
Although some clinicians use the height of the wedge pressure v wave as an indicator of the severity of mitral regurgitation, this practice is fraught with problems and has some fundamental physiologic limitations.[456] [457] [458] [459] [460] Because wedge pressure is a damped reflection of left
A closer look at the left atrial pressure-volume relationships helps explain the apparently paradoxical coexistence of severe mitral regurgitation and a normal PAWP trace.[457] [462] Three factors determine whether mitral regurgitation produces a prominent v wave in the left atrial or wedge pressure traces: left atrial volume (often termed the patient's "volume status"), left atrial compliance, and regurgitant volume ( Fig. 32-40 ). Given that the left atrial pressure-volume relationship is curvilinear, the same volume of regurgitation will result in a variable increment in systolic pressure, depending on the preexisting atrial volume at the onset of systole. Similarly, the shape of the left atrial pressure-volume curve, which reflects atrial stiffness or compliance, will determine the height of the pressure wave for any given regurgitant volume. Accordingly, patients with acute mitral regurgitation tend to have tall wedge pressure v waves because they have smaller, stiffer left atria than do patients with chronic valvular regurgitation. Although the total regurgitant volume of blood entering the left atrium will influence the height of the v wave, this is clearly not the only determinant of v wave magnitude. Therefore, it is not surprising that wedge pressure v waves are neither sensitive nor specific indicators of the severity of mitral regurgitation.[457] [458] [459] [460] Prominent wedge pressure v waves may exist in the absence of mitral regurgitation when left atrial pressure is high, as might occur when the left atrium is compressed.[463] Tall v waves are also seen commonly in patients with hypervolemia, congestive heart failure, and ventricular septal defects.[457] Note that the giant v waves observed in patients with ventricular septal defects are not caused by retrograde flow, but rather by excessive antegrade systolic flow into the left atrium from the left-to-right shunt, which increases pulmonary blood flow and systolic atrial filling through the pulmonary veins.[456]
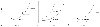 Figure 32-40
Height of the v wave as an indicator of the severity
of mitral regurgitation. Left atrial pressure-volume curves describe the three factors
that determine the height of the v wave. A, Influence
of left atrial volume. For the same regurgitant volume (x), the left atrial v wave
will be taller if baseline atrial volume is greater (point B versus point A). B,
Influence of left atrial compliance. For the same regurgitant volume (x), the left
atrial v wave will be taller if baseline atrial compliance is reduced (point B versus
point A). C, Influence of regurgitant volume. Beginning
at the same baseline left atrial volume (points A and B), if regurgitant volume increases
(X versus x), the left atrial pressure v wave will increase (V versus v). (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 17-13.)
Figure 32-40
Height of the v wave as an indicator of the severity
of mitral regurgitation. Left atrial pressure-volume curves describe the three factors
that determine the height of the v wave. A, Influence
of left atrial volume. For the same regurgitant volume (x), the left atrial v wave
will be taller if baseline atrial volume is greater (point B versus point A). B,
Influence of left atrial compliance. For the same regurgitant volume (x), the left
atrial v wave will be taller if baseline atrial compliance is reduced (point B versus
point A). C, Influence of regurgitant volume. Beginning
at the same baseline left atrial volume (points A and B), if regurgitant volume increases
(X versus x), the left atrial pressure v wave will increase (V versus v). (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 17-13.)
In contrast to mitral regurgitation, which distorts the systolic portion of the wedge pressure waveform, mitral stenosis alters the morphology of the diastolic portions of this waveform. In this condition, the holodiastolic pressure gradient across the mitral valve results in an increased mean wedge pressure, a slurred early diastolic y descent, and a tall end-diastolic a wave. Similar hemodynamic abnormalities are seen in patients with left atrial myxoma or whenever there is obstruction to mitral flow. Diseases that increase left ventricular stiffness (e.g., left ventricular infarction, pericardial constriction, aortic stenosis, and systemic hypertension) produce changes in wedge pressure that resemble in part those seen in mitral stenosis. In these conditions, mean wedge pressure is increased and the trace displays a prominent a wave, but the y descent remains steep because of the absence of obstruction to flow across the mitral valve during diastole. Because patients with advanced mitral stenosis often have coexisting atrial fibrillation, the a wave will not be present in many of these cases, although the other hemodynamic features persist ( Fig. 32-41 ).[312]
Myocardial ischemia is accompanied by a number of physiologic abnormalities that are detectable with the PAC. Ischemia impairs left ventricular relaxation and thereby results in diastolic dysfunction. This pattern of ischemia is particularly characteristic of the demand ischemia associated with tachycardia or induced by rapid atrial pacing.[295] [464] [465] [466] [467] [468] [469] Impaired ventricular relaxation results in a stiffer, less compliant left ventricle in diastole, which leads to increased left ventricular end-diastolic pressure. Not only does this, in turn, increase left atrial and wedge pressure, but the morphology of these waveforms changes as well, with the phasic a and v wave components becoming more prominent as diastolic filling pressure increases.[467] [470] [471] [472] [473] [474] [475] [476] Although myocardial ischemia will often be detectable as a rise in pulmonary artery diastolic, mean, or systolic pressure, these changes are generally less striking than the accompanying change in wedge pressure and the new appearance of tall a and v waves ( Fig. 32-42 ). In patients with left ventricular ischemia, the tall wedge pressure a wave is produced by
 Figure 32-41
Mitral stenosis. Mean pulmonary artery wedge pressure
(PAWP) is increased (35 mm Hg), and the diastolic y descent is markedly attenuated.
Compare the slope of the y descent in the PAWP trace with the y descent in the central
venous pressure (CVP) trace. In addition, compare this PAWP y descent with the PAWP
y descent in mitral regurgitation (see Fig.
32-38
and Fig. 32-39
).
Because of atrial fibrillation, a waves are not seen in the PAWP or CVP traces.
ART, arterial blood pressure. (Redrawn from Mark JB: Atlas of Cardiovascular
Monitoring. New York, Churchill Livingstone, 1998, Fig. 17-19.)
Figure 32-41
Mitral stenosis. Mean pulmonary artery wedge pressure
(PAWP) is increased (35 mm Hg), and the diastolic y descent is markedly attenuated.
Compare the slope of the y descent in the PAWP trace with the y descent in the central
venous pressure (CVP) trace. In addition, compare this PAWP y descent with the PAWP
y descent in mitral regurgitation (see Fig.
32-38
and Fig. 32-39
).
Because of atrial fibrillation, a waves are not seen in the PAWP or CVP traces.
ART, arterial blood pressure. (Redrawn from Mark JB: Atlas of Cardiovascular
Monitoring. New York, Churchill Livingstone, 1998, Fig. 17-19.)
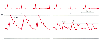 Figure 32-42
Myocardial ischemia. Pulmonary artery pressure (PAP)
is relatively normal and mean pulmonary artery wedge pressure (PAWP) is only slightly
elevated (15 mm Hg). However, PAWP morphology is markedly abnormal, with tall a
waves (21 mm Hg) resulting from the diastolic dysfunction seen in this condition.
(Redrawn from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill
Livingstone, 1998, Fig. 12-4.)
Figure 32-42
Myocardial ischemia. Pulmonary artery pressure (PAP)
is relatively normal and mean pulmonary artery wedge pressure (PAWP) is only slightly
elevated (15 mm Hg). However, PAWP morphology is markedly abnormal, with tall a
waves (21 mm Hg) resulting from the diastolic dysfunction seen in this condition.
(Redrawn from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill
Livingstone, 1998, Fig. 12-4.)
Myocardial ischemia produces a characteristic pattern of left ventricular systolic dysfunction in addition to the diastolic abnormalities noted earlier. Systolic dysfunction is the hallmark of supply ischemia caused by a sudden reduction or cessation of coronary blood flow to a region of the myocardium.[466] [478] With severe systolic dysfunction, changes in global left ventricular contractile performance may be detected with hemodynamic monitoring. As the ejection fraction falls and left ventricular end-diastolic volume and pressure rise, hemodynamic monitoring will show systemic arterial hypotension and elevated pulmonary diastolic and wedge pressure. This hemodynamic pattern is uncommon during anesthesia and surgery and suggests severe systolic myocardial dysfunction. [479] [480] [481] A more common hemodynamic manifestation of myocardial ischemia occurs when left ventricular geometry is distorted or when the region of ischemic myocardium underlies a papillary muscle.[482] Acute mitral valve regurgitation may result, not because of any inherent abnormality of the mitral valve leaflets, but rather because of critical alterations in the supporting structures of the mitral valve, including the mitral annulus, chordae tendineae, papillary muscles, and underlying left ventricular myocardium.[482] [483] This form of mitral regurgitation is often termed "papillary muscle ischemia" or "functional mitral regurgitation." As noted earlier, PAC monitoring is particularly well suited to detect this event by revealing the onset of new regurgitant v waves in the pulmonary artery or wedge pressure trace (see Fig. 32-38 and Fig. 32-39 ).
Whether the PAC should be used in high-risk patients as a supplemental monitor for detection of myocardial ischemia remains uncertain.[484] [485] [486] [487] However, if physicians choose this form of monitoring, they should have a clear understanding of the mechanism by which ischemia alters pulmonary artery and wedge pressure and an appreciation for the anticipated pressure waveform changes. None of the current methods of detecting perioperative myocardial ischemia is perfectly sensitive or specific. Although patients with left ventricular ischemia are likely to have higher mean wedge pressure than those without ischemia, these differences are small and may be difficult to detect clinically.[476] Furthermore, clear quantitative threshold values for mean wedge pressure or a and v wave peak pressures that are diagnostic of ischemia have not been identified, perhaps because of the wide variation in the normal range of these pressures. Consequently, when a PAC is used to diagnose myocardial ischemia, the best approach is to integrate the PAC data with other monitored values and use changes in pulmonary artery or wedge pressure as parts of the diagnostic puzzle.[467] [488]
Right ventricular ischemia produces characteristic hemodynamic patterns that may be recognized with PAC monitoring. Just as left ventricular ischemia increases PAWP, right ventricular ischemia increases CVP, and this is one of the few situations in which CVP may be higher than wedge pressure. In addition, CVP waveform morphology changes in a characteristic manner and displays a prominent a wave resulting from right ventricular diastolic dysfunction and a prominent v wave resulting from ischemia-induced tricuspid regurgitation.[11] [306] [467] [489] [490] The CVP waveform in this condition is described as having an M or W configuration, which refers to the tall a and v waves and steep x and y descents. Severe pulmonary artery hypertension may also result in secondary right ventricular ischemia and dysfunction and increased CVP, but this condition is distinguished from primary right ventricular dysfunction in that PAP and the calculated PVR are not increased in the latter condition.
The CVP waveform in right ventricular infarction shares many morphologic features with that recorded from patients with restrictive cardiomyopathy or pericardial constriction, including elevated mean pressure, prominent a and v waves, and steep x and y descents.[489] [491] The pathophysiologic feature common to these conditions is impaired right ventricular diastolic compliance and is often termed "restrictive physiology." In pericardial constriction, this arises from the restraining effect of the diseased pericardium, whereas in restrictive cardiomyopathy and right ventricular infarction, diastolic dysfunction impairs ventricular relaxation and increases intrinsic ventricular stiffness. Pericardial constriction, also termed constrictive pericarditis or pericardial restriction, limits cardiac filling because of the rigid, often calcified pericardial shell. Impaired venous return decreases end-diastolic volume, stroke volume, and cardiac output. Despite reduced cardiac volumes, cardiac filling pressures are markedly elevated and equal in all four chambers of the heart at end-diastole ( Fig. 32-43 ). Although PAC monitoring reveals this pressure equalization, the characteristic M or W configuration is more apparent in the CVP trace than in the PAWP trace, most likely because of the damping effect of the pulmonary vasculature on the left-sided filling pressure as recorded by the PAC.[167] [492] [493] [494]
Another hemodynamic hallmark of pericardial constriction is observed in the right and left ventricular pressure traces. These traces demonstrate rapid, but short-lived early diastolic ventricular filling, which produces a diastolic "dip-and-plateau" pattern or "square root sign" ( Fig. 32-44 ).[166] [495] In some cases, particularly when the heart rate is slow, a similar waveform pattern may be noted in the CVP trace: a steep y descent (the diastolic dip) produced by rapid early diastolic flow from the atrium to the ventricle, followed by a mid-diastolic h wave (the plateau) from the interruption in flow imposed by the restrictive pericardial shell (see Fig. 32-43 and Fig. 32-44 ).
Like pericardial constriction, cardiac tamponade impairs cardiac filling, but in the case of tamponade, a compressive pericardial fluid collection produces this effect. This filling defect results in a marked increase in CVP and reduced cardiac diastolic volume, stroke volume, and cardiac output. Despite many similar hemodynamic features, tamponade and constriction may be distinguished by the
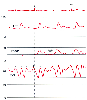 Figure 32-43
Pericardial constriction. This condition causes elevation
and equalization of diastolic filling pressures in the pulmonary artery pressure
(PAP), pulmonary artery wedge pressure (PAWP), and central venous pressure (CVP)
traces. The CVP waveform reveals tall a and v waves with steep x and y descents
and a mid-diastolic plateau wave (asterisk) or h
wave. ART, arterial blood pressure. (Redrawn from Mark JB: Atlas of Cardiovascular
Monitoring. New York, Churchill Livingstone, 1998, Fig. 18-1.)
Figure 32-43
Pericardial constriction. This condition causes elevation
and equalization of diastolic filling pressures in the pulmonary artery pressure
(PAP), pulmonary artery wedge pressure (PAWP), and central venous pressure (CVP)
traces. The CVP waveform reveals tall a and v waves with steep x and y descents
and a mid-diastolic plateau wave (asterisk) or h
wave. ART, arterial blood pressure. (Redrawn from Mark JB: Atlas of Cardiovascular
Monitoring. New York, Churchill Livingstone, 1998, Fig. 18-1.)
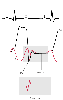 Figure 32-44
Pericardial constriction. The diastolic filling abnormality
in this condition inscribes a "dip-and-plateau pattern" or "square root sign" in
both the right ventricular (RV) and right atrial (RA) pressure traces. (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 18-3.)
Figure 32-44
Pericardial constriction. The diastolic filling abnormality
in this condition inscribes a "dip-and-plateau pattern" or "square root sign" in
both the right ventricular (RV) and right atrial (RA) pressure traces. (Redrawn
from Mark JB: Atlas of Cardiovascular Monitoring. New York, Churchill Livingstone,
1998, Fig. 18-3.)
Probably the single most important waveform abnormality or interpretive problem in PAC monitoring is discerning the correct pressure measurement in patients receiving positive-pressure mechanical ventilation or those who have labored spontaneous respiration. When any pulmonary artery or wedge pressure recording is used to estimate ventricular preload, the physician must consider the confounding effects of changes in intrathoracic pressure that occur during the respiratory cycle. Just as in the case of CVP monitoring, transmural cardiac filling pressures are estimated best when end-expiratory pressure values are recorded (see the earlier section "Physiologic Considerations for Central Venous Pressure Monitoring: Diastolic Pressure-Volume Relationships and Transmural Pressure"). During positive-pressure mechanical ventilation,
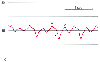 Figure 32-45
Cardiac tamponade. The central venous pressure waveform
shows an increased mean pressure (16 mm Hg) and attenuation of the y descent. Compare
with Figure 32-43
and Figure
32-44
. (Redrawn from Mark JB: Atlas of Cardiovascular Monitoring.
New York, Churchill Livingstone, 1998, Fig. 18-5.)
Figure 32-45
Cardiac tamponade. The central venous pressure waveform
shows an increased mean pressure (16 mm Hg) and attenuation of the y descent. Compare
with Figure 32-43
and Figure
32-44
. (Redrawn from Mark JB: Atlas of Cardiovascular Monitoring.
New York, Churchill Livingstone, 1998, Fig. 18-5.)
|
|
|
|
|
|
|
|
|
|
|
|
|