|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The pattern of effects of volatile anesthetics on cerebral physiology is a striking departure from that observed with the intravenous agents, which generally cause parallel reductions in CMR and CBF. All volatile anesthetics, similar to intravenous sedative-hypnotic drugs, suppress cerebral metabolism in a dose-related manner.[22] [283] [284] [285] [286] [287] Volatile anesthetics also possess intrinsic cerebral vasodilatory activity because of direct effects on vascular smooth muscle. The net effect of volatile anesthetics on CBF is therefore a balance between a reduction in CBF caused by CMR suppression and augmentation of CBF as a result of direct cerebral vasodilation. When administered in a dose of 0.5 MAC, the CMR suppression-induced reduction in CBF predominates, and net CBF decreases in comparison to the awake state. At 1.0 MAC, CBF remains unchanged; at this dose, CMR suppression and vasodilatory effects are in balance. Beyond 1.0 MAC, the vasodilatory activity predominates, and CBF increases significantly even though CMR is substantially reduced.
The increase in CBF produced by volatile anesthetics at doses greater than 1.0 MAC has been interpreted as evidence of uncoupling of flow and metabolism. However, considerable evidence indicates that coupling (CBF adjustments paralleling changes in CMR) persists during anesthesia with volatile anesthetics.[28] [284] [288] [289] [290] [291] [292] Accordingly, it is probably more accurate to say that the CBF/CMR ratio is altered (increased) by volatile anesthetics. This alteration is dose related, and under steady-state conditions, there is a positive correlation between MAC multiples and the CBF/CMRO2 ratio[38] [285] [293] [294] ; that is, higher MAC levels cause greater "luxury" perfusion.
The important clinical consequences of administration of volatile anesthetics are derived from the increases that can occur in CBF and CBV and, consequently, ICP. Of the commonly used volatile anesthetics, the order of vasodilating potency is approximately halothane ≫ enflurane > desflurane ≅ isoflurane > sevoflurane. [29] [287] [291] [295] [296] [297] [298]
Volatile anesthetics possess intrinsic vasodilatory activity, and they not only modify cerebral autoregulation but also produce dose-dependent reductions in systemic blood pressure. Hence, their effects on CBF and CMR are best evaluated when arterial pressure is supported to a common level. In addition, the cerebrovascular effects of volatile anesthetics are modulated by the simultaneous administration of other central nervous system (CNS)-active drugs. It is therefore important to understand the control state (awake, sedated, or anesthetized) against which the CBF and CMR effects of volatile anesthetics are compared. The best information about the cerebrovascular effects of volatile anesthetics is obtained from studies in which a nonanesthetized awake control state is used.
Data on the cerebrovascular effects of halothane and enflurane are limited. Initial studies in humans demonstrated that administration of 1 MAC halothane significantly increased CBF in comparison to preanesthetic CBF values, even when systemic blood pressure was substantially reduced.[299] The same investigators subsequently showed that in humans, when MAP is maintained at 80 mm Hg, 1.1 MAC levels of halothane increased CBF by as much as 191% and decreased CMR by about 10%[295] [299] ( Fig. 21-7 ). When compared with awake values, 1.2 MAC enflurane also increased CBF and decreased CMR by 45% and 15%, respectively.[303] The dramatic increases in CBF with a simultaneous modest reduction in CMR attest to the cerebral vasodilatory properties of halothane and enflurane. Isoflurane, by contrast, does not increase CBF
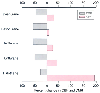 Figure 21-7
Estimated changes in cerebral blood flow (CBF) and the
cerebral metabolic rate of oxygen (CMRO2
) caused by volatile anesthetics.
The CBF data for halothane, enflurane, and isoflurane were obtained during 1.1 minimum
alveolar concentration (MAC) anesthesia (with blood pressure support) in humans[295]
and are expressed as the percent change from awake control values. The CMRO2
data for halothane, enflurane, and isoflurane were obtained from cats[285]
[300]
and are expressed as the percent change from
N2
O-sedated control values. Data for sevoflurane were obtained during
1.1 MAC anesthesia in rabbits and are expressed as the percent change from a morphine/N2
O-anesthetized
control state.[287]
CBF values were obtained in
patients who received 1 MAC sevoflurane anesthesia.[301]
Desflurane data were obtained in patients to whom 1 MAC desflurane was administered.
[302]
Figure 21-7
Estimated changes in cerebral blood flow (CBF) and the
cerebral metabolic rate of oxygen (CMRO2
) caused by volatile anesthetics.
The CBF data for halothane, enflurane, and isoflurane were obtained during 1.1 minimum
alveolar concentration (MAC) anesthesia (with blood pressure support) in humans[295]
and are expressed as the percent change from awake control values. The CMRO2
data for halothane, enflurane, and isoflurane were obtained from cats[285]
[300]
and are expressed as the percent change from
N2
O-sedated control values. Data for sevoflurane were obtained during
1.1 MAC anesthesia in rabbits and are expressed as the percent change from a morphine/N2
O-anesthetized
control state.[287]
CBF values were obtained in
patients who received 1 MAC sevoflurane anesthesia.[301]
Desflurane data were obtained in patients to whom 1 MAC desflurane was administered.
[302]
More recent investigations have shown that both sevoflurane and desflurane can significantly reduce CBF in humans in comparison to CBF values in awake nonanesthetized patients. In 1.0 MAC concentrations, sevoflurane[301] and desflurane[302] decreased CBF by 38% and 22% and CMR by 39% and 35%, respectively. These results, which suggest that the cerebral vasodilation produced by isoflurane is greater than that produced by sevoflurane and desflurane, were obtained with CBF measured by the inert gas technique. This technique measures CBF primarily within the cortex and may therefore have substantially underestimated global CBF. In addition, other investigations in humans, most using transcranial Doppler measurement of MCA flow velocity, indicate that the differences in the effects of isoflurane, desflurane ( Fig. 21-8 ), and sevoflurane are, at best, modest.[291] [298] [316] It should be noted that a strictly quantitative comparison between these volatile anesthetics is not possible given the variations in blood pressure among study group patients. In addition, there is some discrepancy among studies in the literature regarding the magnitude of the effects of volatile anesthetics on CBF.
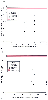 Figure 21-8
Effect of volatile anesthetics on cerebral blood flow
(CBF) (A) and the cerebral metabolic rate of oxygen
(CMRO2
) (B) in humans. The results are
a composite of CBF and CMR values obtained from a number of separate investigations.
[175]
[297]
[301]
[302]
[304]
[305]
[306]
[307]
[308]
[309]
[310]
[311]
[312]
[313]
[314]
[315]
In these studies, PaCO2
was maintained in the normocapnic range (≅35 to 40 mm Hg) and mean arterial pressure
was supported. In most of the investigations, CBF was measured by the inert gas
technique. This technique measures primarily cortical CBF and, as such, might underestimate
global CBF.
Figure 21-8
Effect of volatile anesthetics on cerebral blood flow
(CBF) (A) and the cerebral metabolic rate of oxygen
(CMRO2
) (B) in humans. The results are
a composite of CBF and CMR values obtained from a number of separate investigations.
[175]
[297]
[301]
[302]
[304]
[305]
[306]
[307]
[308]
[309]
[310]
[311]
[312]
[313]
[314]
[315]
In these studies, PaCO2
was maintained in the normocapnic range (≅35 to 40 mm Hg) and mean arterial pressure
was supported. In most of the investigations, CBF was measured by the inert gas
technique. This technique measures primarily cortical CBF and, as such, might underestimate
global CBF.
All the volatile anesthetics cause reductions in CMR. The degree of CMRO2 reduction that occurs at a given MAC level is less with halothane than with the other four agents (see Fig. 21-7 ). Sevoflurane's effect on CMRO2 is very similar to that of isoflurane (see Fig. 21-7 ). [287] The available information, derived from separate investigations, suggests that desflurane causes slightly less suppression of CMRO2 than isoflurane does, especially at concentrations above 1.0 MAC.[22] [286] Although a direct comparison of the CMRO2 effects of all the volatile anesthetics has not been performed in humans, collation of data from a number of investigations has shown that in 1.0 MAC doses, isoflurane, sevoflurane, and desflurane reduce CMRO2 (AVDO2 difference in arterial and jugular bulb blood samples) by 25%,[304] 38%,[301] and 22%,[317] respectively. PET studies in humans have also shown that halothane (0.9 MAC) and isoflurane (0.5 MAC) can decrease the cerebral metabolic rate of glucose (CMRg) by 40% and 46%, respectively.[175] [318] The CMRO2 reduction is dose related. With isoflurane (and almost certainly desflurane and sevoflurane as well), maximal reduction is attained simultaneously with the occurrence of EEG suppression.[22] [286] This reduction occurs at clinically relevant concentrations (i.e., 1.5 to 2.0 MAC) in humans.[291] [319] In dogs, administration of additional isoflurane up to 6.0% end-tidal volume results in no further reduction in CMR and no indication of metabolic toxicity.[22] Halothane presents a contrast to this pattern. Halothane concentrations in excess of 4.0 MAC are required to achieve EEG isoelectricity in dogs, and additional halothane causes a further reduction in CMRO2 in concert with alterations in energy charge. The latter changes, which are reversible, suggest interference with oxidative phosphorylation.[283] These data indicate that unlike isoflurane, halothane can produce reversible toxicity when administered in very high concentrations.
The CBF and CMR dose-response relationships are somewhat alinear for volatile anesthetics. The initial appearance of an EEG pattern associated with the onset of anesthesia with halothane, enflurane, and isoflurane is accompanied by a precipitous decline in CMRO2 .[164] Thereafter, CMRO2 declines in a slower dose-dependent manner. Other studies during anesthetic induction with halothane have observed marked increases in CBF before any alteration in CMR.[165] This finding suggests that the direct effect of a volatile anesthetic on smooth muscle may develop more rapidly than influences related to depression of CMR.
The regional distribution of anesthetic-induced changes in CBF and CMR differ markedly with halothane and isoflurane. Halothane produces relatively homogeneous changes throughout the brain. CBF is globally increased and CMR is globally depressed. The changes caused by isoflurane are more heterogeneous. CBF increases are greater in the subcortical areas and hindbrain structures than in the neocortex. [28] [29] [320] For CMR the converse is true, with a greater reduction in the neocortex than the subcortex.[27] In humans, 1.0 MAC sevoflurane ( Fig. 21-9 ) results in a reduction in CBF within the cortex and an increase in CBF within the cerebellum.[305] These effects of sevoflurane are similar to those produced by isoflurane.[29] [305] Desflurane has not been submitted to similar local CBF studies. However, given the similarity of its effects on the
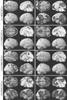 Figure 21-9
Dose-dependent redistribution of cerebral blood flow
(CBF) in humans. Positron emission tomography (PET) scans demonstrate a dose-dependent
reduction in CBF in both sevoflurane-anesthetized (left)
and propofol-anesthetized subjects (right). During
sevoflurane anesthesia, an increase in concentration from 1.5 to 2.0 minimum alveolar
concentration (MAC) leads to an increase in CBF within the subcortex, particularly
in the cerebellum. A gradual reduction in mean arterial pressure (MAP) was observed
with increasing concentrations of sevoflurane, and MAP was not supported. The CBF
values would be expected to be considerably greater had blood pressure been maintained
within the normal range. Therefore, the CBF values represented in the figure probably
underestimate true CBF during sevoflurane anesthesia. In propofol-anesthetized subjects,
CBF was uniformly decreased and redistribution of CBF was not observed. (Data
from Kaisti K, Metsahonkala L, Teras M, et al: Effects of surgical levels of propofol
and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with
positron emission tomography. Anesthesiology 96:1358–1370, 2002.)
Figure 21-9
Dose-dependent redistribution of cerebral blood flow
(CBF) in humans. Positron emission tomography (PET) scans demonstrate a dose-dependent
reduction in CBF in both sevoflurane-anesthetized (left)
and propofol-anesthetized subjects (right). During
sevoflurane anesthesia, an increase in concentration from 1.5 to 2.0 minimum alveolar
concentration (MAC) leads to an increase in CBF within the subcortex, particularly
in the cerebellum. A gradual reduction in mean arterial pressure (MAP) was observed
with increasing concentrations of sevoflurane, and MAP was not supported. The CBF
values would be expected to be considerably greater had blood pressure been maintained
within the normal range. Therefore, the CBF values represented in the figure probably
underestimate true CBF during sevoflurane anesthesia. In propofol-anesthetized subjects,
CBF was uniformly decreased and redistribution of CBF was not observed. (Data
from Kaisti K, Metsahonkala L, Teras M, et al: Effects of surgical levels of propofol
and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with
positron emission tomography. Anesthesiology 96:1358–1370, 2002.)
The data presented thus far, in sum, indicate that isoflurane, desflurane, and sevoflurane may have a modest cerebral vasodilating effect in the human cortex when administered in doses of 1 MAC or less. In fact, as shown in Figure 21-8 , administration of volatile anesthetics can effect net decreases in CBF. These data, however, should be interpreted with considerable caution because the critical variable that is of interest in the clinical sphere is CBV. Although there is a direct correlation between CBF and CBV, as noted earlier, the relationship is not strictly 1:1. The magnitude of the changes in CBV is significantly less than changes in CBF, and a modest reduction in CBF may not necessarily be accompanied by a reduction in CBV. This relationship is exemplified by clinical investigations in which a significant increase in ICP (and by extension, CBV) was observed in patients to whom isoflurane was administered in doses that would be expected to effect a reduction in CBF.[321] [323] Although the induction of hypocapnia mitigated the increase in ICP in these studies, other investigations have revealed that hyperventilation may not be effective in blunting isoflurane-induced increases in ICP in patients with intracranial tumors. [322] In experimental investigations of cerebral injury, volatile anesthetics increased ICP significantly, and this rise in ICP was not ameliorated by hypocapnia.[324] Collectively, these data suggest that volatile anesthetics will have minimal effects on cerebral hemodynamics in patients with normal intracranial compliance. However, in patients with abnormal intracranial compliance, the potential for volatile anesthetic-induced increases in CBV and ICP exists. Accordingly, one should use discretion when administering volatile anesthetics in the setting of large mass lesions, unstable ICP, or sufficient derangement of cerebral physiology that CO2 responsiveness and flow-metabolism coupling may be impaired in some or all of the brain. When they occur (a somnolent, vomiting patient with papilledema, a large mass, and compressed basal cisterns), the clinician may be well advised to use a predominantly intravenous technique until such time as the cranium and dura are open and the effect of the anesthetic technique can be directly assessed. These events will be relatively rare in elective neurosurgery.
Situations in which there has been substantial antecedent lowering of CMR by drug administration or disease processes should also justify caution in the use of volatile anesthetics. If a volatile anesthetic has a substantial direct vasodilating effect on the cerebral vasculature that is normally offset by an opposing metabolically mediated vasoconstricting influence, it might be surmised that when a near-maximal reduction in CMR has occurred, introduction of the volatile anesthetic will have a predominantly vasodilating effect.[180] [289] There are data to support this prediction. Murphy and coworkers[295] observed no increase in CBF (versus awake control measurements) during anesthesia with 0.6 and 1.1 MAC isoflurane (with blood pressure support), but at 1.6 MAC, CBF had increased by 100%. Maekawa and colleagues[27] measured CBF and local CMRg in rats both awake and during anesthesia with increasing concentrations of isoflurane. Isoflurane at 1 MAC resulted in an average CMRg decrease of 54% of the awake control value in five cortical areas and no change in average CBF. An additional 1.0 MAC (i.e., a total of 2.0 MAC) caused a further CMRg reduction of only 20% of the control value, and CBF simultaneously increased by 70% ( Fig. 21-10 ). These data together suggest that isoflurane is a significant cerebral vasodilator when administered in concentrations at
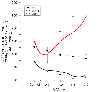 Figure 21-10
Relationship between changes in the cerebral metabolic
rate for glucose (CMRg) and cerebral blood flow (CBF) in the motor-sensory cortex
in rats during isoflurane anesthesia. Most of the CMR suppression caused by isoflurane
had occurred by 1.0 minimum alveolar concentration (MAC), and in this concentration
range CBF is not increased. Thereafter, additional isoflurane causes little further
reduction in CMR, and cerebral vasodilation occurs. These data (±SD), from
Maekawa and colleagues,[27]
suggest the importance
of metabolic coupling in determining the CBF effects of isoflurane.
Figure 21-10
Relationship between changes in the cerebral metabolic
rate for glucose (CMRg) and cerebral blood flow (CBF) in the motor-sensory cortex
in rats during isoflurane anesthesia. Most of the CMR suppression caused by isoflurane
had occurred by 1.0 minimum alveolar concentration (MAC), and in this concentration
range CBF is not increased. Thereafter, additional isoflurane causes little further
reduction in CMR, and cerebral vasodilation occurs. These data (±SD), from
Maekawa and colleagues,[27]
suggest the importance
of metabolic coupling in determining the CBF effects of isoflurane.
The net vasodilating effect of equi-MAC concentrations of isoflurane, desflurane, and sevoflurane is less in humans than that of halothane, and the former are probably therefore preferable if a volatile anesthetic is to be used in the setting of impaired intracranial compliance. That is not to say that halothane is contraindicated in these circumstances. It has clearly been demonstrated that when hypocapnia is established before the introduction of halothane, the increases in ICP that might otherwise occur in a normocapnic patient with poor intracranial compliance can be prevented or greatly attenuated.[325] Nonetheless, most clinicians will prefer isoflurane, desflurane, or sevoflurane because the margin for error is probably wider than with halothane.
The effect of volatile anesthetics on CBF has been shown to be time dependent in animal investigations. After an initial increase, CBF falls substantially, with a steady state near pre-volatile anesthetic levels reached between 2½ and 5 hours after exposure.[46] [320] [326] The mechanism of this effect is not understood, and the phenomenon was not evident in humans studied during 3- or 6-hour exposure to halothane, isoflurane, desflurane, or sevoflurane.[291] [327]
The extensive investigation of the influence of volatile anesthetics on CBF has been based primarily on the concern that cerebral vasodilation, produced by volatile anesthetics, might increase ICP. It should be noted, however, that it is CBV and not CBF per se that influences ICP. The vast majority of intracranial blood is within the cerebral venous circulation, and although there is a reasonable correlation between vasodilation-induced increases in CBF and CBV, the magnitude of CBF changes is considerably greater than the changes in CBV ( Fig. 21-11 ). Hence, changes in CBF do not reliably predict changes in CBV and, by extension, in ICP. Nonetheless, the available data do indicate that CBV is considerably greater during isoflurane anesthesia than during propofol or pentobarbital anesthesia. [136] In addition, CBV responds to changes in PaCO2 by a reduction in CBV with hypocapnia and an increase in CBV with hypercapnia.[328] The magnitude of the change in CBV is, however, less than the change in CBF.
CO2 responsiveness is well maintained during anesthesia with all of the volatile anesthetics.[38] [297] [329] [330] [331] By contrast, autoregulation of CBF in response to rising arterial pressure is impaired. This impairment appears to be most apparent with anesthetics that cause the greatest cerebral vasodilation[285] [295] and is dose related (see Fig. 21-5 ). [332] [333] Sevoflurane may cause less impairment in autoregulation than other volatile anesthetics do. Recent studies surprisingly reported no change in CBFV in response to phenylephrine-induced MAP increases during anesthesia with 1.2 to 1.5 MAC sevoflurane[331] [334] [335] or in CBF during hemorrhagic hypotension.[336] The autoregulatory response to rising pressure is, however, rarely of significance in clinical neuroanesthesia. If anything, it is the
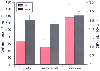 Figure 21-11
Effect of anesthetics on cerebral blood flow (CBF) and
cerebral blood volume (CBV). When compared with isoflurane, propofol and pentobarbital
effected substantial reductions in CBF. However, CBV reductions were more modest.
[136]
These data indicate that the magnitude of
the effect of anesthetic agents on CBF is substantially greater than the effect on
CBV. Hence, a reduction in CBF may not lead to equivalent reductions in CBV.
Figure 21-11
Effect of anesthetics on cerebral blood flow (CBF) and
cerebral blood volume (CBV). When compared with isoflurane, propofol and pentobarbital
effected substantial reductions in CBF. However, CBV reductions were more modest.
[136]
These data indicate that the magnitude of
the effect of anesthetic agents on CBF is substantially greater than the effect on
CBV. Hence, a reduction in CBF may not lead to equivalent reductions in CBV.
Enflurane is potentially epileptogenic in the clinical setting. Of particular relevance to neuroanesthesia is the observation that hypocapnia potentates seizure-type discharges during enflurane anesthesia.[337] A 50% decrease in CMRO2 was noted in human volunteers anesthetized with 3% enflurane, but with the onset of seizure activity, CMRO2 returned to normal,[338] thus indicating preservation of flow-metabolism coupling. Note that there is no evidence that this type of EEG activity is deleterious when oxygen delivery is maintained during the event. However, because seizure activity can elevate brain metabolism by as much as 400%, the use of enflurane, especially in high doses and with hypocapnia, should probably be avoided in patients who are predisposed to seizures or have occlusive cerebrovascular disease.
The EEG-activating property of enflurane has been used intraoperatively to activate and identify seizure foci that are to be surgically resected, and in this situation, spike activity not present preoperatively has been observed to persist after surgery.[339] In addition, two reports have described seizures in the immediate postoperative period after enflurane anesthesia in both predisposed[340] and nonpredisposed individuals. [341] No apparent permanent sequelae have resulted from these events, and in fact, this association is not a rigorously proven one. At worst, such occurrences are extremely uncommon.
Isoflurane can cause EEG spiking and myoclonus, but in the experimental setting it has not been associated with the frank epileptoid activity induced by enflurane.
Seizures have been reported to occur during induction of anesthesia with high concentrations of sevoflurane in children, including those without a recognized seizure diathesis.[345] [346] In two healthy human subjects, EEG burst suppression with 2 MAC sevoflurane was accompanied by epileptiform discharges that were observed during EEG monitoring. [347] These discharges were associated with a significant increase in CBF, thus demonstrating that flow and metabolism coupling was preserved. In patients with temporal lobe epilepsy, the administration of 1.5 MAC sevoflurane elicited widespread paroxysmal interictal EEG activity. Of note was the observation that paroxysmal activity was not restricted to the ictal focus and that the administration of sevoflurane was not of any assistance in localization of the epileptogenic region of the brain.[348] The development of tonic-clonic movements indicative of seizure activity has also been reported in otherwise healthy patients on emergence from sevoflurane anesthesia.[349] [350] In all of the reported cases of seizure activity associated with sevoflurane anesthesia, untoward sequelae have not been documented. These reports highlight sevoflurane's ability, albeit small, to evoke epileptiform activity, and accordingly, the use of sevoflurane in patients with epilepsy should be undertaken with appropriate caution.
The available data unequivocally indicate that N2 O can cause increases in CBF, CMR, and ICP. At least a portion of the CBF and CMR increases may be the result of a sympathoadrenal stimulating effect of N2 O. [351] The magnitude of the effect varies considerably depending on the presence or absence of other anesthetics ( Fig. 21-12 ). When N2 O is administered alone, very substantial increases in CBF and ICP can occur. In sharp contrast, when N2 O is administered in combination with intravenous drugs, including barbiturates, benzodiazepines, narcotics, and propofol, its cerebral vasodilating effect is attenuated or even completely inhibited. The addition of N2 O to anesthesia induced with a volatile anesthetic will result in moderate CBF increases.
The most dramatic reported increases in ICP or CBF in humans[354] [355] and experimental animals[351] [356] [357] have occurred when N2 O was administered alone or with minimal background anesthesia. For instance, Henriksen and Jorgensen[354] recorded ICP before and during spontaneous breathing of 66% N2 O by patients with intracranial tumors. Mean ICP rose from 13 to 40 mm Hg. The CBF increases observed in humans are more modest than those observed in animals, but they are still substantial. [352] Whether these substantial increases represent the effects of N2 O per se or whether they reflect the nonspecific effects of "second-stage" arousal phenomena is not known.
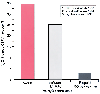 Figure 21-12
Mean percent increases in cerebral blood flow volume
(CBFV) in the middle cerebral artery of normocapnic subjects exposed to 60% N2
O
after control recording in three conditions: awake,[352]
1.1 minimum alveolar concentration (MAC) isoflurane,[290]
and propofol, 150 µg/kg/min.[353]
Figure 21-12
Mean percent increases in cerebral blood flow volume
(CBFV) in the middle cerebral artery of normocapnic subjects exposed to 60% N2
O
after control recording in three conditions: awake,[352]
1.1 minimum alveolar concentration (MAC) isoflurane,[290]
and propofol, 150 µg/kg/min.[353]
When N2 O is administered in conjunction with certain intravenous anesthetics, its CBF effect may be considerably reduced. Phirman and Shapiro[358] observed that a reproducible increase in ICP that had occurred in response to administration of 70% N2 O to a comatose patient was prevented by previous administration of a combination of pentothal and diazepam in spite of no change in baseline ICP. In an investigation of patients with intracranial tumors and poor intracranial compliance (mean preinduction ICP, 27 mm Hg),[359] 50% N2 O introduced during barbiturate anesthesia and after the induction of hypocapnia had a negligible effect on ICP. Jung and colleagues compared lumbar CSF pressure in patients with brain tumors during the administration of 0.7% isoflurane or 70% N2 O after induction of anesthesia with a barbiturate. Lumbar CSF pressure was modestly, but significantly greater with N2 O.[360] That the increase was less dramatic than those cited earlier for N2 O alone may reflect the presence of residual barbiturate. Benzodiazepines administered alone blunt the CBF response to N2 O in both animals and humans.[361] [362] Narcotics appear to have a similar effect. Jobes and coauthors[152] reported that anesthesia with 1 mg/kg of morphine plus 70% N2 O resulted in no change in CBF from awake control values. Because of the very minor effect of morphine on CBF, these data suggest that N2 O did not cause substantial cerebral vasodilation. In a study using transcranial Doppler, Eng and associates observed no change in CBF velocity when N2 O was introduced in patients anesthetized with propofol. [353]
In most investigations, including several in humans, in which N2 O has been added to an anesthetic of 1.0 MAC or greater,
The results of three investigations, including one in humans, indicate that this vasodilating effect of N2 O may be positively correlated with the concentration of inhaled anesthetic[365] [366] [369] and suggest that in general, the CBF increase caused by N2 O is exaggerated at higher concentrations of both halothane and isoflurane.
No uniform agreement regarding the effect of N2 O on CMR has not reached. Parallel changes in CBF and CMR,[351] [356] CBF increases without an alteration in CMR, [368] [371] [372] and CMR alteration occurring without changes in CBF[373] have all been reported. This variability is doubtless the product of differences in species, methods, depth of background anesthesia, and interactions with simultaneously administered drugs. Few data derived from humans are available. Wollman and coauthors [373] reported that 70% N2 O caused a 15% reduction in CMRO2 in human volunteers. However, both premedication and induction of anesthesia were accomplished with barbiturates, and the control group was nonconcurrent. Algotsson and coworkers[370] reported no difference in CMRO2 . From animal experimentation it appears likely that at least in some circumstances, N2 O causes increases in CMR. The "cleanest" investigation is that of Pelligrino and colleagues,[356] who measured cortical CBF and CMR in awake goats that received N2 O without premedication or other supplements. After 60 minutes of N2 O inhalation, cortical CMRO2 was increased to 170% and global CBF to 143% of control values. The goats showed no evidence of an excitement stage. They became lethargic and experienced a fall in plasma epinephrine levels suggesting the absence of stress.
The CBF response to CO2 is preserved during administration of N2 O.[366] [368] [373]
In spite of the inconsistencies that are evident, data indicate that the vasodilatory action of N2 O can be clinically significant in neurosurgical patients with reduced intracranial compliance.[354] However, it appears that N2 O-induced cerebral vasodilation can be considerably blunted by the simultaneous administration of intravenous anesthetics, although not all anesthetics have been evaluated and the dose-response relationships are not well defined. N2 O has been widely used in neurosurgery, and banishing it is inconsistent with the accumulated experience. Nonetheless, in circumstances wherein ICP is persistently elevated or the surgical field is persistently "tight," N2 O should be viewed as a potential contributing factor. In addition, the ability of N2 O to rapidly enter a closed gas space should be recalled, and it should be avoided or omitted when a closed intracranial gas space may exist.
|
|
|
|
|
|
|
|
|
|
|
|
|