|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quality, safety, efficiency, and the cost of drugs and equipment are all important considerations in choosing an anesthetic technique for outpatient surgery.[217] The ideal outpatient anesthetic should have a rapid and smooth onset of action, produce intraoperative amnesia and analgesia, provide good surgical conditions with a short recovery period, and have no adverse effects. Outpatient surgery requires the same basic equipment as inpatient surgery for delivery of anesthetic drugs, monitoring, and resuscitation. Standard intraoperative monitoring equipment for outpatient operations should include an ECG, blood pressure cuff, pulse oximeter, and capnograph. If nondepolarizing muscle relaxants are used, a neuromuscular monitor should also be available. Increasingly, cerebral monitors are also being used to improve titration of anesthetic drugs and facilitate faster recovery.
The choice of anesthetic technique depends on both surgical and patient factors. For many ambulatory procedures, general anesthesia remains the most popular technique with both patients and surgeons. Although central neuraxis blockade has traditionally been popular for peripheral extremity and lower abdominal procedures, its use in the ambulatory setting can delay discharge because of residual motor and sympathetic blockade. Peripheral nerve blocks facilitate the recovery process by minimizing the need for postoperative opioid analgesics. Therefore, an increasing number of ambulatory cases are being performed with a combination of local anesthetic nerve blocks and intravenous sedation (so-called monitored anesthesia care [MAC]). Although there is no ideal anesthetic drug or technique for outpatients, a vast array of pharmacologically active drugs, when combined in a rational manner and carefully titrated, can produce the desired anesthetic conditions with an acceptable recovery profile and reasonable cost.
The ability to deliver a safe and cost-effective general anesthetic with minimal side effects and rapid recovery is critical in a busy outpatient surgery unit.[217] Despite a higher incidence of side effects, general anesthesia remains the most widely used anesthetic technique for managing ambulatory surgery. The use of heated airway humidifiers,[218] forced air warmers,[219] and passive heat and moisture exchangers will further decrease fluid losses and conserve heat during longer outpatient procedures. For major laparoscopic surgery, devices that heat and humidify insufflation gases may improve maintenance of core body temperature while reducing postoperative pain.[220] Interestingly, using a forced air warming device with standard hospital blankets was found to be as effective in maintaining body temperature as a commercial forced air heating blanket.[221] For ambulatory procedures lasting less than 90 minutes, forced warming devices are unlikely to be cost-effective.[219]
Tracheal intubation causes a high incidence of postoperative airway-related complaints, including sore throat, croup, and hoarseness (see Chapter 42 ). Most outpatients undergoing superficial procedures under general anesthesia do not require tracheal intubation unless they are at high risk for aspiration. The laryngeal mask airway (LMA) was first introduced in 1983 as an alternative to tracheal intubation or a facemask for airway management. When compared with anesthesia with a mask and oral airway, patients with an LMA had fewer desaturation episodes, fewer intraoperative airway manipulations, and fewer difficulties in maintaining an airway.[222] The incidence of postoperative sore throat after ambulatory surgery was 18% with an LMA versus 45% with a tracheal tube and 3% with a face mask.[223] The LMA frees the anesthesiologist's hands for record keeping, monitoring, and drug administration. Hand fatigue from maintaining the airway with a mask is also eliminated.
The LMA can be positioned easily without direct visualization
or neuromuscular blocking drugs, and the patient can be allowed to ventilate spontaneously
throughout the procedure.[224]
Although desflurane
is more pungent than sevoflurane, the LMA is equally well tolerated during the maintenance
period with both volatile anesthetics.[225]
When
compared with tracheal intubation, insertion of the LMA causes minimal cardiovascular
responses and is better tolerated at lighter levels of anesthesia. The incidence
of
|
|
Dose (mg/kg) | Onset of Action | Recovery Profile | Side Effects |
|---|---|---|---|---|
| Thiopental | 3–6 | Rapid | Intermediate | Drowsiness ("hangover") |
| Methohexital | 1.5–3 | Rapid | Rapid | Pain, excitatory activity |
| Etomidate | 0.15–0.3 | Rapid | Intermediate | Pain, myoclonus, emesis |
| Ketamine | 0.75–1.5 | Immediate | Intermediate | Psychomimetic reactions, cardiovascular stimulation |
| Midazolam | 0.1–0.2 | Slow | Slow | Drowsiness, amnesia |
| Propofol | 1.5–2.5 | Rapid | Rapid | Pain on injection, cardiovascular depression |
| From White PF: Ambulatory anesthesia and surgery: Past, present, and future. In White PF (ed): Ambulatory Anesthesia and Surgery. London, WB Saunders, 1997. | ||||
Induction of general anesthesia is typically accomplished with a rapid-acting intravenous anesthetic ( Table 68-8 ) (see Chapter 10 ). Propofol has virtually replaced the barbiturates for induction of anesthesia in the ambulatory setting because of its favorable recovery profile.[232] [233] However, the most popular technique for maintenance of anesthesia is a combination of a volatile anesthetic and nitrous oxide. The extremely low solubility of nitrous oxide and the newer volatile anesthetics (i.e., sevoflurane and desflurane) contributes to a more rapid onset and recovery from general anesthesia. Although it has been suggested that the use of nitrous oxide is associated with PONV, controlled studies have questioned the clinical importance of nitrous oxide in producing this side effect.[234] [235] [236] [237] When compared with a target-controlled infusion of propofol for maintenance of anesthesia, the use of desflurane or sevoflurane produced similar anesthetic conditions with shorter emergence times and at a lower drug cost.[238] [239] [240] [241]
Thiopental (3 to 6 mg/kg) is the prototypical intravenous induction drug with a rapid onset and a relatively short
Although midazolam (0.2 to 0.4 mg/kg IV) has been used for induction of anesthesia in outpatients, its onset of action is slower and recovery is prolonged in comparison to the barbiturate compounds and propofol.[245] When combined with nitrous oxide and a potent opioid analgesic, lower doses of midazolam (0.1 to 0.15 mg/kg) can be used to induce general anesthesia. If midazolam is used for induction and flumazenil, a specific benzodiazepine antagonist, is administered at the end of surgery, prompt recovery can be achieved after outpatient surgery. [246] When compared with propofol, recovery after flumazenil antagonism of midazolam anesthesia offered no clinically significant advantages.
Etomidate (0.2 to 0.3 mg/kg) has also been used for induction and maintenance (1 to 3 mg/min) of general anesthesia during short outpatient procedures. [247] Recovery tends to be faster than after thiopental and compares favorably with methohexital. Disadvantages of etomidate include pain on injection, a high incidence of PONV, myoclonic movements, and transient suppression of adrenal steroidogenesis.[247] [248] Given its side effect profile, the use of etomidate should be restricted to clinical situations in which its hemodynamic stability offers a distinctive advantage over the other available induction drugs (e.g., elderly outpatients with clinically significant coronary artery or cerebrovascular disease).
Ketamine is a unique sedative-analgesic that can be used for both induction and maintenance of general anesthesia.[249] However, ketamine compares unfavorably with both the barbiturates and propofol for minor gynecologic procedures because of its prominent psychomimetic effects and higher incidence of PONV during the early postoperative period.[250] Use of the more potent S(+)-isomer of ketamine may decrease some of the adverse effects associated with the racemic mixture in the ambulatory setting.[102] [251] Premedication with a benzodiazepine (e.g., midazolam, 0.05 mg/kg IV) decreases the incidence of ketamine-induced emergence reactions. [249] Small doses of ketamine (10 to 20 mg IV) have been used as an alternative to potent opioid analgesics during induction of anesthesia with propofol.[252] Furthermore, adjunctive use of 75 to 150 µg/kg intravenously during ambulatory surgery produced an opioid-sparing effect after painful orthopedic procedures.[253] [254]
Propofol is an intravenous anesthetic with an extremely high metabolic clearance rate (see Chapter 10 ). [255] Recovery after propofol anesthesia compares favorably with the other intravenous anesthetics in the outpatient setting.[256] [257] [258] Although propofol costs more than the barbiturate anesthetics do, the use of propofol may contribute to significant savings because of decreased recovery costs.[96] Recovery after induction of anesthesia with propofol is faster than with the barbiturate compounds,[258] irrespective of the maintenance drug ( Fig. 68-5 ).[139] [233] [243] [257] When compared with methohexital, the use of propofol was associated with fewer perioperative side effects (e.g., less hiccoughs, nausea, and vomiting) and faster overall recovery times.[232] The faster intermediate recovery with propofol-based anesthetics may also yield significant savings in nursing costs (e.g., less overtime). For example, a 15-minute reduction in the phase I recovery room stay could save 1000 nursing hours in a 4,000-case per year ambulatory surgical facility. [259] Interestingly, the use of a single induction dose of propofol may facilitate earlier discharge after ambulatory surgery, irrespective of the maintenance anesthetic.[260]
The use of propofol is associated with less PONV and a decreased requirement for antiemetic medications.[232] [256] [261] [262] [263] [264] There is evidence that propofol possesses inherent antiemetic activity, and it has even been
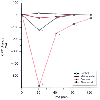 Figure 68-5
Comparison of mean changes in choice reaction time (CRT)
postoperatively in untreated controls and outpatients who received thiopental, methohexital,
or propofol for induction of anesthesia. *P
< .05. (Redrawn with modification from Mackenzie N, Grant IS: Comparison
of the new emulsion formulation of propofol with methohexitone and thiopentone for
induction of anaesthesia in day cases. Br J Anaesth 57:725, 1985.)
Figure 68-5
Comparison of mean changes in choice reaction time (CRT)
postoperatively in untreated controls and outpatients who received thiopental, methohexital,
or propofol for induction of anesthesia. *P
< .05. (Redrawn with modification from Mackenzie N, Grant IS: Comparison
of the new emulsion formulation of propofol with methohexitone and thiopentone for
induction of anaesthesia in day cases. Br J Anaesth 57:725, 1985.)
Volatile anesthetics are most commonly used for maintenance of ambulatory anesthesia (see Chapter 5 , Chapter 6 , Chapter 7 , Chapter 8 , and Chapter 9 ). Changes in the depth of anesthesia can be achieved readily because of the rapid uptake and elimination of these anesthetics.[269] [270] The rapid elimination of anesthetic vapors provides for fast patient recovery and potentially earlier discharge from the outpatient facility. Although a similar spectrum of pharmacologic activity is produced by all of the available volatile anesthetics, isoflurane was the most commonly used anesthetic for maintenance of ambulatory anesthesia before the introduction of the less soluble drugs sevoflurane and desflurane. For procedures lasting longer than 90 minutes, recovery times were faster after isoflurane than after halothane and enflurane.[271] Most studies involving pediatric patients have reported that halothane is associated with the lowest incidence of perioperative complications.[272] However, ventricular arrhythmias are more likely to occur during induction with halothane than with sevoflurane.
The newer volatile anesthetic sevoflurane is a useful alternative
to halothane for inhalation induction in the outpatient setting because of its lack
of pungency and more favorable recovery profile ( Table
68-9
).[273]
[274]
[275]
[276]
[277]
[278]
When used for maintenance of anesthesia,
immediate
recovery (emergence) after desflurane was significantly faster than with isoflurane
( Table 68-10
).[279]
Emergence from
| Drug Name | Concentration (%) | Onset of Action | Recovery Profile | Side Effects |
|---|---|---|---|---|
| Halothane | 0.5–1.5 | Slow | Slow | Sedation, arrhythmias |
| Enflurane | 0.75–1.5 | Intermediate | Intermediate | Shivering |
| Isoflurane | 0.5–1 | Intermediate | Intermediate | Coughing |
| Desflurane | 3–6 | Very rapid | Very rapid | Coughing, tachycardia |
| Sevoflurane | 1–2 | Rapid | Rapid | Flammable |
| Nitrous oxide | 50–70 | Very rapid | Very rapid | Nausea/emesis (?) |
| From White PF: Ambulatory anesthesia and surgery: Past, present, and future. In White PF (ed): Ambulatory Anesthesia and Surgery. London, WB Saunders, 1997. | ||||
|
|
Isoflurane | Desflurane |
|---|---|---|
| Elimination half-time (min) | 9.5 ± 3.4 | 2.5 ± 0.8 * |
| Opening eyes | 10.2 ± 77 | 5.1 ± 2.4 * |
| Following commands | 11.1 ± 7.9 | 6.5 ± 2.3 |
| Sitting up in a chair | 113 ± 27 | 95 ± 56 |
| PACU discharge | 118 ± 36 | 105 ± 49 |
| Home-ready (discharge) | 231 ± 40 | 207 ± 54 |
| PACU, postanesthesia care unit. | ||
| From Ghouri AF, Bodner M, White PF: Recovery profile after desflurane-nitrous oxide vs isoflurane-nitrous oxide in outpatients. Anesthesiology 74:419, 1991. | ||
When compared with the newer volatile anesthetics, propofol anesthesia offered the advantage of a lower incidence of PONV ( Fig. 68-7 ).[284] [285] Importantly, early comparative studies of propofol, sevoflurane, and desflurane were performed before the introduction of fast-track recovery paradigms involving the routine use of multimodal therapies for minimizing pain and emesis. As a result of its low solubility (0.42), desflurane is associated with the most rapid recovery of cognitive and psychomotor function.[283] [285] [286] [287] [288] [289] [290] [291] In morbidly obese patients, both emergence and intermediate recovery times were consistently more rapid after desflurane than after propofol or isoflurane.[292] In addition, desflurane was associated with better postural control than propofol was in the early recovery period.[293] Clinical studies have shown a greater incidence of postoperative agitation and excitement in patients recovering from sevoflurane and desflurane versus the traditional volatile anesthetics. Furthermore, desflurane can produce significant autonomic stimulation after rapid adjustments in its inspired concentration.[287] [288] [294] [295] The pungency of inhaled desflurane precludes its use in high concentration for induction of anesthesia.[296]
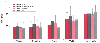 Figure 68-6
Intermediate recovery after propofol, desflurane, and
propofol-desflurane anesthesia. (Redrawn from Van Hemelrijck J, Smith I,
White PF: Use of desflurane for outpatient anesthesia: A comparison with propofol
and nitrous oxide. Anesthesiology 75:197, 1991.)
Figure 68-6
Intermediate recovery after propofol, desflurane, and
propofol-desflurane anesthesia. (Redrawn from Van Hemelrijck J, Smith I,
White PF: Use of desflurane for outpatient anesthesia: A comparison with propofol
and nitrous oxide. Anesthesiology 75:197, 1991.)
Sevoflurane has lower solubility than halothane does and an impressive lack of airway irritation, thus making it a very useful alternative for induction of anesthesia in adults.[297] [298] [299] [300] [301] In the elderly, induction with sevoflurane was also associated with better hemodynamic stability than induction with propofol was.[302] Even inhalational induction using a single-breath technique is well tolerated with sevoflurane because of its lack of pungent odor. As a result, sevoflurane has become popular for both pediatric and adult outpatient anesthesia.[303] [304] [305] [306] [307] [308] However, when compared with propofol and halothane, sevoflurane was associated with an increased incidence of adverse events during induction and recovery (e.g., delirium and PONV). [309] [310] Although sevoflurane can be degraded in carbon dioxide absorbents to compound A, clinical studies have failed to demonstrate significant changes in renal or hepatic function when sevoflurane was used at low gas flow rates (1 L/min) or during closed-circuit anesthesia.[17] [311] [312] [313]
Volatile anesthetics are associated with a higher incidence of vomiting in the early recovery period than propofol-based anesthetic techniques are.[17] [314] However, delayed
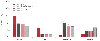 Figure 68-7
Postoperative nausea after propofol, desflurane, and
propofol-desflurane anesthesia. (Redrawn from Van Hemelrijck J, Smith I,
White PF: Use of desflurane for outpatient anesthesia: A comparison with propofol
and nitrous oxide. Anesthesiology 75:197, 1991.)
Figure 68-7
Postoperative nausea after propofol, desflurane, and
propofol-desflurane anesthesia. (Redrawn from Van Hemelrijck J, Smith I,
White PF: Use of desflurane for outpatient anesthesia: A comparison with propofol
and nitrous oxide. Anesthesiology 75:197, 1991.)
Opioid compounds are frequently administered in the immediate preinduction period to suppress autonomic responses to tracheal intubation and during the maintenance period to treat acute autonomic responses to painful (noxious) stimuli (see Chapter 11 ). Opioids can
The use of small doses of potent opioids (e.g., fentanyl, 1 to 2 µg/kg, alfentanil, 15 to 30 µg/kg, sufentanil, 0.15 to 0.3 µg/kg, or remifentanil, 0.5 to 1 µg/kg) can effectively attenuate the cardiostimulatory response to laryngoscopy and intubation, as well as the skin incision. These drugs are also useful supplements to inhaled anesthetics during the maintenance period. When compared with a standard inhaled volatile anesthetic, most investigators have demonstrated improved intraoperative conditions and more rapid emergence from anesthesia when fentanyl or one of its newer analogs was administered as part of a balanced anesthetic technique.[322] [323] When a sufentanil infusion was compared with fentanyl for maintenance of general anesthesia in combination with nitrous oxide, its use was associated with less nausea and postoperative pain.[324] Because alfentanil has a more rapid onset and shorter duration of action than fentanyl does, emergence and recovery of psychomotor function are faster after an anesthetic technique based on alfentanil (versus fentanyl).[323] Alfentanil has been alleged to cause less PONV than equipotent doses of fentanyl or sufentanil does in outpatients.[325]
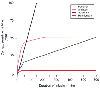 Figure 68-8
Computer simulation of the "context-sensitive" half-life
time (CSHT) for remifentanil (3.65 minutes), alfentanil (58.5 minutes), sufentanil
(240 minutes), and fentanyl (262.5 minutes). Note that remifentanil's CSHT is independent
of the duration of infusion. (Redrawn with modification from Egan TD, Lemmens
HJ, Fiset P, et al: The pharmacokinetics of the new short-acting opioid remifentanil
(G187084B) in healthy adult male volunteers. Anesthesiology 79:881, 1993.)
Figure 68-8
Computer simulation of the "context-sensitive" half-life
time (CSHT) for remifentanil (3.65 minutes), alfentanil (58.5 minutes), sufentanil
(240 minutes), and fentanyl (262.5 minutes). Note that remifentanil's CSHT is independent
of the duration of infusion. (Redrawn with modification from Egan TD, Lemmens
HJ, Fiset P, et al: The pharmacokinetics of the new short-acting opioid remifentanil
(G187084B) in healthy adult male volunteers. Anesthesiology 79:881, 1993.)
Remifentanil is an ultrashort-acting opioid analgesic with potency similar to that of fentanyl. It is rapidly metabolized by nonspecific tissue esterases, a process that allows for rapid systemic elimination, with a half-life of 8 to 10 minutes and a context-sensitive half-life (the time for a 50% decrease in its effect site concentration after discontinuing an infusion) of 4 minutes regardless of the duration of infusion ( Fig. 68-8 ). [326] [327] [328] In contrast, the context-sensitive half-life of alfentanil is 58 minutes because it depends on its small volume of distribution for rapid termination of its clinical effect.[329] When remifentanil was compared with alfentanil as part of a total intravenous anesthesia technique, remifentanil provided more effective suppression of intraoperative responses but led to prolonged recovery room stay because of increased postoperative pain.[330] Studies involving the use of remifentanil in combination with the less soluble volatile anesthetics suggest that a low-dose infusion (0.05 to 0.2 µg/kg/min) can produce a significant anesthetic-sparing effect and thereby contribute to faster emergence from anesthesia.[331] [332] Furthermore, a 0.5- to 1-µg/kg bolus dose of remifentanil was more effective than a standard dose of fentanyl in suppressing the acute hemodynamic response to laryngoscopy and tracheal intubation in patients undergoing outpatient laparoscopy. [119] Smaller doses of remifentanil (0.25 µg/kg) can facilitate placement of an LMA device.[333] In elderly outpatients, total intravenous anesthesia with remifentanil and propofol was associated with more rapid recovery of psychomotor function than a standard fentanyl-isoflurane technique.[334] However, remifentanil is less cost-effective than either fentanyl or alfentanil when administered as an adjuvant to general anesthesia because of drug wastage.[335] [336]
Morphine, hydromorphone, oxymorphone, and meperidine have all been used in outpatient anesthesia. However, these opioid compounds are less popular than the more potent, rapid, and shorter-acting opioid analgesics (e.g., fentanyl, sufentanil, alfentanil, and remifentanil). When intraoperative morphine and fentanyl were compared in ambulatory surgical patients, higher postoperative pain scores and greater oral analgesic use were found in the fentanyl group.[337] However, morphine was associated with increased nausea and vomiting in the postdischarge period. Motion-induced emesis is a major concern when morphine and its more lipophobic congeners are used in the ambulatory setting.
The semisynthetic opioid agonist-antagonist compounds (e.g., butorphanol, nalbuphine, dezocine, tramadol) have a theoretical advantage over the potent opioid compounds as adjuvants during general anesthesia because of their limited potential to produce ventilatory depression. Unfortunately, there is also a "ceiling effect" with respect to their analgesic efficacy. Although intraoperative use of dezocine provided significantly longer postoperative analgesia than fentanyl did, it increased postoperative nausea and delayed discharge after outpatient anesthesia.[338] When nalbuphine (0.3 to 0.5 mg/kg) was compared with fentanyl (1.5 µg/kg) during outpatient anesthesia, it was found to produce more unpleasant dreaming during surgery and greater postoperative anxiety, drowsiness, and emetic symptoms.[122]
Many superficial outpatient surgical procedures do not require the use of neuromuscular relaxants. However, muscle relaxants are commonly used during laparoscopic surgery, ophthalmologic ("open globe") and ENT procedures, and operations performed in the prone position. When remifentanil is used in combination with propofol for induction of anesthesia, tracheal intubation can be performed without any muscle relaxants.[339] [340] Nevertheless, muscle relaxants are still commonly used to facilitate tracheal intubation and to optimize surgical conditions while minimizing the use of anesthetic and analgesic drugs. Although it has been suggested that controlled ventilation with muscle relaxants may decrease postoperative emesis, this effect was not verified when controlled (versus spontaneous) ventilation was compared in outpatients undergoing strabismus repair. [341]
Succinylcholine remains the most commonly used muscle relaxant to facilitate tracheal intubation in the ambulatory setting because it has a rapid onset and its short duration of action obviates the need for reversal drugs. Before the introduction of short- and intermediate-acting nondepolarizing muscle relaxants, [342] an infusion of succinylcholine was the most frequently used muscle relaxant technique during outpatient laparoscopic surgery. Although the administration of succinylcholine has been alleged to cause myalgia after surgery, the use of mivacurium or vecuronium as alternatives to succinylcholine has failed to lower the incidence or severity of muscle pain after laparoscopy.[342] [343] Pretreatment with small doses of rocuronium before succinylcholine also failed to reduce the incidence or severity of postoperative myalgia and is not recommended because it may cause uncomfortable muscle weakness before loss of consciousness.[344]
Use of the short- and intermediate-acting nondepolarizing muscle relaxants (e.g., cisatracurium, mivacurium) allows reversal of neuromuscular blockade even after brief surgical procedures.[345] An intubating dose of mivacurium (0.15 to 0.20 mg/kg) has approximately twice the duration of action of succinylcholine (20 to 30 minutes) but a significantly more rapid spontaneous recovery profile than atracurium, vecuronium, or rocuronium does.[346] [347] A cost-effective technique involves the use of succinylcholine for tracheal intubation followed by small (4 to 8 mg) bolus doses of mivacurium during the maintenance period. This relaxant technique minimizes the need for muscle relaxant reversal drugs after short laparoscopic procedures.[342]
Pharmacologic antagonists (i.e., drugs that compete with agonists at specific receptor sites) may be useful in facilitating the recovery process after ambulatory anesthesia. However, antagonists may also produce unwanted side effects (e.g., dizziness, headaches, PONV) that should be considered before routinely using these drugs. In addition, because their duration of action is often shorter than the agonist (e.g., naloxone, flumazenil), a "rebound" of the agonist effect may occur. This latter effect is a concern in the ambulatory setting because patients are frequently discharged home within 2 hours after surgery.
Opioid-induced rigidity and respiratory depression can be treated with small incremental boluses of naloxone. Because naloxone has its own intrinsic side effects (e.g., nausea and vomiting, hypertension, pulmonary edema, and arrhythmias), [348] it is obviously preferable to titrate the opioid carefully to achieve the desired effect during surgery without the need for reversal drugs. For treating opioid-induced muscle rigidity, small doses of succinylcholine (5 to 10 mg IV) are also highly effective without adversely affecting the opioid's analgesic action.
The central effects of benzodiazepines (e.g., residual sedation and amnesia) can be promptly reversed with flumazenil (0.2 to 0.5 mg IV). Although flumazenil is a specific benzodiazepine antagonist, its use also leads to an increase in side effects. As with opioids, it is more cost-effective to carefully titrate the benzodiazepines to the desired clinical effect rather than relying on flumazenil to reverse residual depressant effects. Resedation can occur 1 to 2 hours after reversal because the half-life of flumazenil is shorter than that of the benzodiazepine agonists.[349] [350]
Intermediate-acting neuromuscular drugs are usually reversed at the end of surgery, with neostigmine and edrophonium being the two most widely used anti-cholinesterase drugs. Several studies have found that the choice of the reversal drug influences the frequency of PONV in the ambulatory setting.[342] When compared with patients receiving no reversal drugs or reversal with edrophonium, patients receiving neostigmine had an increased frequency of PONV in the early postoperative period.[351] [352] Mivacurium may be advantageous for use during the maintenance period because reversal is seldom required if the drug is properly titrated.[342]
In unruly, frightened, or mentally retarded children, preoperative sedation is required before taking the patient into the operating room (see Chapter 60 ). In general, sedative premedication is not offered to children younger than 12 months, but it is often used for toddlers or preschool-aged children. Because of the aversion of children to needles, oral or rectal premedicants are more popular. Midazolam remains the most commonly used anxiolytic premedication for pediatric outpatients. After receiving 0.5 mg/kg of midazolam orally, children can be easily separated from their parents within 30 minutes without prolonging the discharge time even after short surgical procedures.[108] [353] When rectal methohexital is administered (20 to 30 mg/kg) before volatile anesthesia, recovery times will be prolonged. Rectal etomidate (6 mg/kg) or ketamine (5 to 10 mg/kg) can produce a rapid onset of hypnosis without cardiorespiratory depression in children undergoing outpatient procedures.[354] Although ketamine (2 to 4 mg/kg intramuscularly) can be an extremely useful drug for induction of anesthesia in an uncooperative or mentally retarded child, home readiness is delayed when larger doses of ketamine (>5 mg/kg) are combined with volatile anesthetics.[355] In addition, psychomimetic reactions have been reported in children after ketamine administration.[356]
The practice of allowing parents to be present during induction of anesthesia is becoming more common.
|
|
|
|
|
|
|
|
|
|
|
|
|