|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Optimal preoperative preparation of outpatients makes ambulatory surgery both safer and more acceptable for patients and the hospital staff (see Chapter 25 ). The preparation process is aimed at reducing the risks inherent in ambulatory surgery, improving patient outcome, and making the surgical experience more pleasant for the patient and their family. Simple procedures such as topical warming of an extremity before venous cannulation reduce both the time and number of attempts required.[83] Preparation should minimize patient anxiety through pharmacologic and nonpharmacologic means and reduce potential postoperative problems through the use of appropriate premedication. Patients should be encouraged to continue all their chronic medications up to the time that they arrive at the surgery center. Oral medications can be taken with a small amount of water up to 30 minutes before surgery. Practitioners need to be aware of the fact that an increasing number of outpatients are using nutraceuticals,[84] some of which may cause adverse effects during the perioperative period.[85]
The anticipation of undergoing anesthesia can cause psychological stress that is manifested as anxiety. Preoperative anxiety has been shown to rise at least 1 week before surgery and return to normal levels in the postoperative period only after an uneventful recovery is ensured.[86] The cause of patients' preoperative anxiety is multifactorial, but most commonly it is related to concerns about intraoperative awareness, not awaking after surgery, or postoperative pain and nausea. Not surprisingly, previous surgery reduces the degree of preoperative anxiety.[87] High levels of stress preoperatively are associated with slower recovery and greater analgesic and antiemetic requirements after surgery, but it can be effectively reduced by careful preoperative preparation ( Fig. 68-2 ). [88] Overall, well-informed patients tend to recover faster and experience less pain and fewers postoperative complications.
Nonpharmacologic preparation has many desirable characteristics. These methods are economical, lack undesirable side effects, and are associated with high patient acceptance and motivation. More than 40 years ago, Egbert and colleagues[89] demonstrated that the anesthetist's visit was more effective than a sedative premedicant in relieving preoperative anxiety. Postoperative pain has been similarly reduced by preoperative educational programs. The timing of the preoperative interview is also important because anxiety is significantly decreased only when the interview takes place outside the operating room immediately before surgery.[90] Many centers have shown encouraging results with the use of instructional preoperative videotapes that offer a complete explanation of the events surrounding surgery.[91] Outpatients who listen to music before surgery reported significantly lower anxiety levels.[92] Self-hypnotic relaxation techniques have proved beneficial during invasive medical procedures.[93] Hypnosis may also have a positive effect in reducing pain and anxiety, as well as improving hemodynamic stability during the perioperative period.
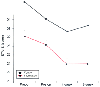 Figure 68-2
Preoperative psychological preparation reduces stress
before and up to 1 week after surgery, as measured by the Spielberger state anxiety
score. *P < .05. (Redrawn with modification
from Wallace LM: Psychological preparation as a method of reducing the stress of
surgery. J Hum Stress 10:62, 1984. Reprinted with permission of the Helen Dwight
Reid Educational Foundation. Published by Heldref Publications, 1319 Eighteenth
St, Washington, DC 20036-1802. Copyright 1984.)
Figure 68-2
Preoperative psychological preparation reduces stress
before and up to 1 week after surgery, as measured by the Spielberger state anxiety
score. *P < .05. (Redrawn with modification
from Wallace LM: Psychological preparation as a method of reducing the stress of
surgery. J Hum Stress 10:62, 1984. Reprinted with permission of the Helen Dwight
Reid Educational Foundation. Published by Heldref Publications, 1319 Eighteenth
St, Washington, DC 20036-1802. Copyright 1984.)
The use of play-oriented preoperative teaching, books, pamphlets, and video programs may be particularly beneficial for pediatric patients.[94] Separation anxiety and postoperative behavioral changes, prominent in children 1 to 4 years of age, may be diminished with such preparation programs. In the modern ambulatory surgery center, both psychological and pharmacologic means should be available to alleviate patients' anxiety and decrease postoperative morbidity.[95] Proper preoperative preparation should also include written and verbal instructions regarding arrival time and place, fasting instructions, and information concerning the postoperative course, limitations in driving skills, and the need for a responsible adult to care for the patient during the postoperative period.
The use of premedication in the outpatient setting has been a subject of considerable debate over the last 20 years. The primary indications for preoperative medication in the outpatient setting are similar to those for patients undergoing inpatient procedures. These indications include anxiolysis, sedation, analgesia, amnesia, vagolysis, and prophylaxis against postoperative emesis and aspiration pneumonia.[96] Despite these well-recognized indications, premedication is not routinely used at many ambulatory surgery facilities in the United States.[2] An important concern in outpatient anesthesia is prompt recovery, and many anesthesiologists avoid using centrally active depressant premedicants because of concern that these drugs will prolong the recovery period. Interestingly, most prospective studies have not found recovery to be prolonged after the use of appropriate
When administered for premedication, sedative-hypnotic drugs can allay anxiety and reduce the overall anesthetic requirements, thereby improving recovery. Traditionally, the most widely used anxiolytic-sedative-hypnotic premedications have been barbiturates and benzodiazepines. These drugs produce dose-related anxiolysis, sedation, and ultimately, unconsciousness. Nonetheless, barbiturates are not commonly used as premedicants in the outpatient setting because of their adverse effects (e.g., residual sedation), which makes them less cost-beneficial than non-barbiturate sedative drugs (e.g., midazolam, propofol).[101] Ketamine has been used for rectal premedication in children. However, side effects were frequent unless it was combined with midazolam.[102] Melatonin has also been reported to produce sedation and anxiolysis comparable to oral midazolam when administered for premedication.[103]
The use of small doses of benzodiazepines for premedication is
a well-established practice. As expected, the amnesic and anxiolytic properties
of these drugs are also useful in the outpatient setting ( Table
68-5
). Although diazepam was the most commonly used oral benzodiazepine,
midazolam has become the drug of choice because its shorter elimination half-life
and lack of significant side effects facilitate the recovery process after ambulatory
surgery.[104]
[105]
To achieve the maximum benefit of administering midazolam for premedication,
|
|
Dosage Range | Onset (min) | Key Points |
|---|---|---|---|
| Benzodiazepines |
|
|
|
| Midazolam | 7.5–15 mg PO | 15–30 | Large first-pass effect |
|
|
5–7 mg IM | 15–30 | Water soluble, nonirritating |
|
|
1–2 mg IV | 1–53 | Rapid onset, excellent amnesia |
| Diazepam | 5–10 mg PO | 45–90 | Long-acting metabolites |
| Temazepam | 15–30 mg PO | 15–40 | Comparable anxiolysis to midazolam |
| Triazolam | 0.125–0.25 mg PO | 15–30 | Prominent sedation |
| Lorazepam | 1–2 mg PO | 45–90 | Prolonged amnestic effect |
| α2 -Adrenergic Agonists |
|
|
|
| Clonidine | 0.1–0.3 mg PO | 45–60 | Prolonged sedative effect |
| Dexmedetomidine | 50–70 µg IM | 20–60 | Bradycardia and hypotension |
|
|
50 µg IV | 5–30 | Reduced anesthetic/analgesic requirements |
| Modified from White PF: Ambulatory anesthesia and surgery: Past, present, and future. In White PF (ed): Ambulatory Anesthesia and Surgery. London, WB Saunders, 1997. | |||
Temazepam and alprazolam have also been reported to be effective oral premedicants for outpatient surgery.[107] [112] Although oral triazolam can produce effective sedation and amnesia, it is less effective than diazepam or midazolam in decreasing preoperative anxiety. Lorazepam, because of its long duration of amnesia, is not routinely used in the ambulatory setting. If a patient expresses significant anxiety during the preoperative interview, an oral benzodiazepine can be prescribed to be taken at home in the evening and in the morning 60 to 90 minutes before surgery. However, a responsible adult must accompany the patient to the surgery center. If significant anxiety develops after admission to the day surgery center, intravenous midazolam (1 to 3 mg) is the most useful drug.
Premedication with α2 -adrenergic agonist drugs can reduce anesthetic and analgesic dosage requirements and produce sedation and anxiolysis while also decreasing the heart rate and blood pressure during anesthesia. Oral clonidine, the prototypical α2 -agonist, has been successfully used for ambulatory premedication. However, residual postoperative sedation may be a problem for elderly outpatients.
Dexmedetomidine is a more highly selective α2 -agonist that has a shorter duration of action than clonidine.[113] Because of its high potency and selective agonist action on α2 -receptors, dexmedetomidine may gain more widespread
Routine use of narcotic (opioid) analgesics for premedication is not recommended unless the patient is experiencing acute pain (see Chapter 11 ). The use of opioid premedicant combinations can increase the incidence of PONV and may result in delayed discharge after ambulatory surgery.[97] The potent opioid analgesics may be beneficial if given intravenously before induction because they can provide acute control of preoperative anxiety, decrease the anesthetic induction dose requirement, minimize the hemodynamic response to airway stimulation, and potentially contribute to postoperative pain relief.[119] However, if reduction of anxiety is the primary goal, it should be achieved with sedative-anxiolytic drugs. It is unclear whether the newer, shorter-acting fentanyl analogs alfentanil and remifentanil offer any significant advantages over the parent compound with respect to perioperative side effects.[120] [121] [122]
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) during
the perioperative period has been studied extensively. However, controversy surrounds
the preoperative use of NSAIDs because of their side effect profile (e.g., antiplatelet
effect) and limited efficacy in controlling acute postoperative pain.[123]
Nevertheless, newer NSAIDs have proved to be valuable analgesic adjuvants as a result
of their long-lasting opioid-sparing actions and lack of effect on platelet function.
When administered as
| Patient-Related Factors |
| Age, gender, preexisting diseases (e.g., diabetes), history of motion sickness or postoperative nausea and vomiting, smoking history, and level of anxiety, as well as intercurrent illness (e.g., viral infection, pancreatic disease) |
| Anesthesia-Related Factors |
| Premedication, opioid analgesics, induction and maintenance anesthetics, reversal (antagonist) drugs, gastric distention, inadequate hydration, and residual sympathectomy |
| Surgery-Related Factors |
| Operative procedure, length of surgery, blood in the gastrointestinal tract, forcing oral intake, opioid analgesics, premature ambulation (postural hypotension), and pain |
| Data from Watcha MF, White PF: Postoperative nausea and vomiting: Its etiology, treatment, and prevention. Anesthesiology 77:162, 1992. |
To achieve the optimal benefit of NSAIDs in the ambulatory setting, they should be administered on a "fixed" dosing schedule beginning in the preoperative period and extending into the postdischarge period.[127] To minimize the potential for operative site bleeding, as well as gastric mucosal and renal tubal toxicity, the more selective cyclooxygenase-2 (COX-2) inhibitors are increasingly being used as alternatives to the classic nonselective NSAIDs during the perioperative period. Oral premedication with rofecoxib (50 mg), celecoxib (400 mg), or valdecoxib (40 mg) reduces pain and the opioid analgesic requirement in the postoperative period, thereby leading to improved patient satisfaction with the quality of recovery after ambulatory surgery.[128] [129] [130] [131] [132] In the future, the parenterally active COX-2 inhibitor parecoxib (20 to 40 mg IV) may prove to be a valuable adjunct in the ambulatory setting.[133] [134] [135]
PONV remains a common problem after general anesthesia and contributes to patient dissatisfaction with the ambulatory surgery experience.[9] [136] When patients undergoing a preanesthetic evaluation were asked which postoperative side effects were of greatest concern, PONV accounted for 49% of the responses.[136] Over 70% of patients considered avoidance of PONV to be very important. In fact, patients are willing to pay a significant amount of money out of pocket to avoid PONV.[137] These symptoms can delay discharge and result in unplanned overnight hospital admission. [11] [30] [138] [139] The choice of anesthetic technique, type of surgery, and use of opioid analgesics can all influence the incidence of PONV. Additional factors that have been reported to influence the incidence of PONV include preexisting medical conditions, gender, pregnancy, phase of the menstrual cycle, type of surgery, preoperative hydration status, postoperative hypotension, and even age ( Table 68-6 ).[140] [141] [142] [143] The incidence of emesis is low in infants, gradually increases
A variety of risk scoring systems have been developed in an attempt to improve the use of antiemetic therapies.[145] [146] Apfel and colleagues[147] identified female gender, nonsmoking status, history of PONV or motion sickness, and postoperative use of opioid analgesics as the major risk factors. This highly simplified risk scoring system provides better discrimination than do the more complex risk scores that have been described.[148] A guideline for the prophylaxis and treatment of PONV based on the estimated risk for PONV has been proposed ( Fig. 68-3 ). [145]
It is unlikely that any single drug will ever be effective in treating emesis under all ambulatory situations. Therefore, a combination of antiemetic drugs is increasingly being used as part of a multimodal regimen.[149] [150] [151] As a result of the efficacy of these combinations, routine antiemetic prophylaxis is being used more frequently in outpatients undergoing a wider variety of ambulatory surgery procedures.[152]
Droperidol is a butyrophenone neuroleptic drug with antiemetic properties as a result of its antagonistic effects at central dopamine receptors. It has been extensively used for the treatment and prevention of PONV over the last 30 years.[153] Numerous studies involving both children and adults have demonstrated that low-dose droperidol is a highly effective antiemetic.[138] [154] Larger doses of droperidol (>20 µg/kg) enhance postoperative sedation and may prolong recovery and discharge in ambulatory surgical patients.[155] Smaller doses of droperidol (<10 µg/kg) can
 Figure 68-3
Algorithm for the prophylaxis and treatment of postoperative
nausea and vomiting. DOLA, dolasetron; DROP, droperidol; OND, ondansetron; PONV,
postoperative nausea and vomiting. (Redrawn from Watcha MF: The cost-effective
management of postoperative nausea and vomiting. Anesthesiology 92:931, 2000.)
Figure 68-3
Algorithm for the prophylaxis and treatment of postoperative
nausea and vomiting. DOLA, dolasetron; DROP, droperidol; OND, ondansetron; PONV,
postoperative nausea and vomiting. (Redrawn from Watcha MF: The cost-effective
management of postoperative nausea and vomiting. Anesthesiology 92:931, 2000.)
The antiemetic action of phenothiazines also results from their ability to block dopamine receptors in the chemoreceptor trigger zone of the brain. Chlorpromazine and promethazine have been used for many years to treat opioid-induced nausea and vomiting.[141] Premedication with phenothiazines can produce intraoperative hypotension along with significant residual sedation after surgery, thus delaying discharge home. In addition, these compounds may produce extrapyramidal effects ranging from restlessness to oculogyric crisis.[158] Therefore, this class of antiemetic is rarely used in the outpatient setting.
Metoclopramide and domperidone are gastrokinetic drugs that facilitate gastric and small-bowel motility and increase lower esophageal sphincter tone. Metoclopramide (20 mg IV or 0.2 mg/kg IV) is effective in the prevention of PONV. Because of its short half-life, metoclopramide should be administered near the end of surgery to ensure optimal efficacy in the early postoperative period.[158] The combination of metoclopramide, 10 to 20 mg intravenously, and droperidol, 0.5 to 1.0 mg intravenously, appears to be more effective than droperidol alone.[150] [157]
Anticholinergic drugs (e.g., atropine, glycopyrrolate, and scopolamine) have traditionally been used for their anti-sialagogue and vagolytic properties. However, transdermal scopolamine, a centrally active anticholinergic, is effective in controlling motion sickness. Preoperative placement of a patch can reduce the incidence of severe PONV, but the patch should be placed several hours before surgery to achieve a therapeutic level in the early postoperative period. When administered before outpatient gynecologic laparoscopy,[159] transdermal scopolamine was associated with a high incidence of adverse effects (95% versus 45%), including dry mouth, somnolence, mydriasis, and dizziness.
Dimenhydrinate and hydroxyzine are antihistaminic compounds that also act on the central vomiting center and vestibular pathways to prevent PONV. They are particularly useful for the prophylaxis and treatment of motion sickness and for outpatients undergoing middle ear surgery. Dimenhydrinate has also been shown to be successful in decreasing vomiting after strabismus surgery.[160] When given in a dose of 0.5 mg/kg at induction of anesthesia, dimenhydrinate significantly reduced vomiting for up to 24 hours and did not delay discharge from the ambulatory facility.[161]
Discovery of the role of 5-hydroxytryptamine (5-HT) in the pathophysiology of drug-induced emesis, as well as the discovery of specific 5-HT antagonists, has generated considerable interest in the use of these compounds for the treatment and prevention of PONV. Ondansetron, granisetron, dolasetron, and tropisetron are all highly selective 5-HT3 receptor antagonists that lack the sedative, dysphoric, or extrapyramidal effects of other commonly used antiemetics.[162] [163] [164] [165] [166] [167] However, these compounds appear to produce headache and are less effective in preventing nausea than other classes of antiemetic drugs are. Ondansetron blocks both central and peripheral 5-HT3 receptors and is effective in preventing emesis after ambulatory surgery.[162] [163] [164] [165] [166] It has also been shown to be effective in the treatment of established postoperative emesis in both adults[163] [167] and children.[168] Because ondansetron has a short elimination half-life, it is more effective in reducing the need for rescue antiemetics in the recovery room when it is administered at the end of longer surgical procedures. [169] [170] The use of small, 1- to 2-mg doses of ondansetron has not been found to be as effective as 4- and 8-mg doses in preventing PONV in the postdischarge period after ambulatory surgery.[163] [170] In a direct comparison of droperidol, 0.625 mg, and ondansetron, 4 mg, the two antiemetics had similar efficacy and discharge times, but droperidol was found to be more cost-effective. [154] [171] This finding has been confirmed in a subsequent multicenter study.[172] Of the available 5-HT3 antagonists, dolasetron appears to be the most cost-effective in both adults[173] and children. [174]
Preliminary studies suggest that the neurokinin-1 (NK-1) antagonists have superior antinausea and longer-lasting antiemetic properties than the 5-HT3 antagonists do.[175] [176] The NK-1 antagonists are effective in both the treatment[175] and prevention[176] of PONV. In addition, these compounds may act synergistically with 5-HT3 antagonists.[176] [177]
Dexamethasone, 4 to 8 mg intravenously, is highly effective in the prevention of PONV when administered alone or in combination with other antiemetic drugs.[178] [179] In addition, both inhaled isopropyl alcohol and transcutaneous capsicum plaster (applied at the standard P6 and Korean K9 acupoints) appear to possess significant antinausea activity.[180] [181] Interestingly, supplemental oxygen does not seem to be effective in reducing PONV after ambulatory surgery.[182]
Acupuncture and acupressure have been used for the prevention of PONV with varying degrees of success.[141] [181] [183] [184] In sham-controlled studies, P6 acupressure compared favorably with metoclopramide.[185] [186] More recently, Korean hand acupressure was shown to be superior to ondansetron in reducing PONV in both adults and children. [181] [187] [188] [189] In outpatients undergoing laparoscopic cholecystectomy procedures,[190] acupressure compared favorably with ondansetron (4 mg IV) in preventing PONV. Recent sham-controlled studies evaluating transcutaneous electrical acupoint stimulation with the ReliefBand device have found that this nonpharmacologic technique was more effective than the 5-HT3 antagonist ondansetron.[191] Interestingly, most studies have suggested that the use of electrostimulation is more successful in preventing nausea than vomiting (or emesis).[191] [192] [193]
Early studies suggested that outpatients may have a theoretically increased risk of aspiration based on the finding that a larger percentage of patients had a residual gastric volume greater than 25 mL with a pH less than 2.5.[194] However, subsequent studies have found no increased risk of aspiration in "fasted" outpatients versus inpatients undergoing elective surgery.[195] Therefore, routine prophylaxis for acid aspiration is no longer recommended. However, in outpatients with predisposing risk factors for pulmonary aspiration (i.e., pregnancy, scleroderma, hiatal hernia, nasogastric tubes, severe diabetics, morbid obesity), the use of H2 receptor blocking drugs or proton pump inhibitors for premedication may be appropriate.
H2 receptor antagonists are effective in increasing gastric pH and decreasing gastric volume by reducing gastric acid secretion ( Table 68-7 ). Ranitidine offers the advantage over cimetidine of a prolonged period of protection and fewer side effects.[196] [198] [199] [200] [201] If given intravenously, ranitidine has a faster onset of action and better protection than cimetidine does.[202] When compared with fasted patients who did not receive ranitidine, patients who received fluids plus oral ranitidine 2 to 3 hours
| Medication (Dose) | Dosing Interval (min) | Gastric Volume (mL) | Gastric pH | Patients with pH <2.5 and Vol >25 mL (%) | Controls with pH <2.5 and Vol >25 mL (%) | References |
|---|---|---|---|---|---|---|
| Ranitidine, 150 mg PO | 113 | 1 | 5.2 | 0 | 35 | Sutherland et al (1986)[196] |
| With 150 mL H2 O | 154 | 10 | 6.4 | 5 | 60 | Manchikanti et al (1985)[113] |
| With 150 mL H2 O | 144 | 8 | 5.5 | 0 | 46 | Maltby et al (1988)[197] |
| With 150 mL coffee | 160 | 14 | 5.7 | 6 | 38 | Maltby et al (1988)[197] |
| With 150 mL juice | 142 | 15 | 5.4 | 8 | 42 | Maltby et al (1988)[197] |
| Ranitidine, 50 mg IV | 60 | 8 | 4.8 | 3 | 20 | Memis et al (2003) |
| Cimetidine, 300 mg PO | 146 | 13 | 5.0 | 4 | 48 | Manchikanti and Rousch (1984)[198] |
| Cimetidine, 400 mg PO | 105 | 13 | 5.0 | 12 | 35 | Stock and Sutherland (1985)[199] |
| Sodium citrate (Bicitra), 15 mL PO, and metoclopramide, 10 mg IV | 50 | 22 | 3.4 | 4 | 36 | Manchikanti et al (1985)[113] |
| Sodium citrate (Bicitra), 30 mL PO, and metoclopramide, 10 mg IV | 50 | 26 | 3.7 | 8 | 36 | Manchikanti et al (1985)[113] |
| Sodium citrate (Bicitra), 15 mL PO | 46 | 58 | 3.7 | 12 | 36 | Manchikanti et al (1985)[113] |
| Sodium citrate (Bicitra), 30 mL PO | 46 | 58 | 3.7 | 12 | 36 | Manchikanti et al (1985)[113] |
| Pantoprazole, 40 mg IV | 60 | 15 | 5.3 | 10 | 20 | Memis et al (2003)[204] |
| From White PF (ed): Ambulatory Anesthesia and Surgery. London, WB Saunders, 1997. | ||||||
Sodium citrate is a nonparticulate antacid that rapidly increases gastric fluid pH. It is commonly used in obstetric and emergency situations because it reduces the consequences of aspiration of gastric contents in high-risk patients. Ingestion of this antacid increases gastric volume, so the addition of a gastrokinetic agent is necessary when administered in the outpatient setting.[205] Disadvantages of its use include the potential for emesis because of its unpleasant taste, a variable duration of effect, and decreased efficacy when compared with H2 receptor antagonists.[206] Sodium citrate's use should be restricted to a select outpatient population (e.g., diabetic or morbidly obese patients) at the highest risk for complications from aspiration of acidic gastric contents.
Metoclopramide, a dopamine antagonist, reduces gastric volume by stimulating gastric emptying without altering pH. The use of effective doses of metoclopramide in combination with an H2 receptor blocking drug has been advocated to decrease postoperative emesis and further reduce the potential risk for aspiration pneumonitis.[207] Other studies have failed to demonstrate a significant advantage of this drug combination over an H2 receptor antagonist alone.[200] However, metoclopramide may offer an additional protective effect as a result of its ability to increase lower esophageal sphincter tone.
Prolonged fasting does not guarantee an empty stomach at the time of induction. Therefore, several investigators have questioned the value of even a 4- to 5-hour fast before elective surgery.[201] [208] [209] [210] Because the half-life of clear fluids in the stomach is 10 to 20 minutes, residual gastric volume after 2 hours is less in patients ingesting small amounts of clear fluids than in fasted patients.[211] After an overnight fast, 50% and 44% of outpatients complained of moderate to severe hunger and thirst, respectively.[196] More important, 14% of young female outpatients arriving at the operating room in the early afternoon after an overnight fast had a serum glucose concentration less than 45 mg/dL.[212] Of interest, ingestion of 150 mL of water 2 hours before surgery significantly decreased the severity of thirst without increasing gastric volume in fasted outpatients.[201] Furthermore, the ingestion of 150 mL of either coffee or orange juice 2 to
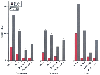 Figure 68-4
Preoperative hydration of 20 versus 2 mL/kg decreases
postoperative morbidity in outpatients. (Redrawn with modification from
Yogendran S, Asokumar B, Cheng DC, et al: A prospective randomized double-blinded
study of the effect of intravenous fluid therapy on adverse outcomes on outpatient
surgery. Anesth Analg 80:682, 1995.)
Figure 68-4
Preoperative hydration of 20 versus 2 mL/kg decreases
postoperative morbidity in outpatients. (Redrawn with modification from
Yogendran S, Asokumar B, Cheng DC, et al: A prospective randomized double-blinded
study of the effect of intravenous fluid therapy on adverse outcomes on outpatient
surgery. Anesth Analg 80:682, 1995.)
Arbitrary restrictions (e.g., nothing by mouth [NPO] after midnight) dictating when outpatients may drink fluids before an elective operation are completely unwarranted. A national survey in the United States demonstrated that 69% of anesthesiologists had changed their NPO guidelines to allow the ingestion of clear fluids for children and 41% had changed their guidelines for adults.[215] Unless outpatients have delayed gastric emptying or receive a narcotic (opioid) premedicant, the requirement for a 10- to 16-hour fast is not justified. Importantly, adequate hydration before induction of anesthesia is associated with a decreased incidence of postoperative side effects, including pain, dizziness, drowsiness, thirst, and nausea ( Fig. 68-4 ). [216]
|
|
|
|
|
|
|
|
|
|
|
|
|