|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The neural membrane is able to maintain a voltage difference of 60 to 90 mV between its inner and outer aspects because at rest it is relatively impermeable to sodium ions but selectively permeable to potassium ions. An active, energy-dependent mechanism, the Na+ /K+ pump, sustains the ion gradients that drive this potential difference by constant extrusion of sodium from within the cell in exchange for a net uptake of potassium by using adenosine triphosphate as an energy source. Although the membrane is relatively permeable to potassium ions, an intracellular-to-extracellular potassium ratio of 150 to 5 mM, or 30:1, is maintained because of both the membranes'
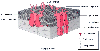 Figure 14-5
A typical plasma membrane has at its core the lipid bilayer,
which is composed of phospholipids and cholesterol molecules (in about a 5:1 ratio)
embedding the membrane integral proteins. These proteins are most often glycosylated
by extracellular carbohydrates and include receptors and ion channels essential for
intercellular communication. "Peripheral proteins" regulate the functions of membrane
proteins, chaperone them to the plasma membrane, and stabilize them in the cell through
interactions with both the cytoskeleton and the extracellular matrix. Probable membrane
locations and protein sites for local anesthetics are also shown.
Figure 14-5
A typical plasma membrane has at its core the lipid bilayer,
which is composed of phospholipids and cholesterol molecules (in about a 5:1 ratio)
embedding the membrane integral proteins. These proteins are most often glycosylated
by extracellular carbohydrates and include receptors and ion channels essential for
intercellular communication. "Peripheral proteins" regulate the functions of membrane
proteins, chaperone them to the plasma membrane, and stabilize them in the cell through
interactions with both the cytoskeleton and the extracellular matrix. Probable membrane
locations and protein sites for local anesthetics are also shown.
The nerve at rest behaves largely as a "potassium electrode,"
according to the Nernst equation:
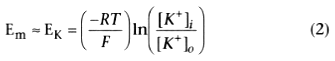
where Em
is the membrane potential, EK
is the potassium equilibrium
potential, R is the gas constant, T is temperature (Kelvin), F is Faraday's constant,
and [K+
] is the potassium ion concentration inside (i) and outside (o)
the cell. For potassium, therefore,
EK
= −58 log 30, or −85.7 mV
An opposite situation exists for Na+ , which is at higher concentration outside the cell and has a Nernst potential, ENa , of about +60 mV. During an action potential the nerve membrane transiently switches its permeability from K+ selective to Na+ selective, thus changing the membrane potential from negative to positive, and back again.[8] The progress of this potential change and the underlying events are graphed in Figure 14-6 . They provide a basis for understanding local anesthetic conduction block.
Ion permeation through membranes occurs by means of special proteins called ion channels.[9] The conformation of these channels is often sensitive to the membrane potential; both Na+ and K+ channels in nerve membranes are activated to an open conformation by membrane depolarization. Sodium channels, in addition, close to an inactivated conformation after their initial activation. A small membrane depolarization, one that extends along an axon from a region of excited membrane, for example, will begin to open both Na+ and K+ channels. The Na+ channels open faster, however, and because the membrane potential is initially much further from the Nernst potential for Na+ than for K+ , the inwardly directed Na+ current is larger ( Fig. 14-6 ). Sodium ions thus entering the nerve depolarize it further, which leads to the opening of more Na+ channels and thereby increases the current even further ( Fig. 14-7 ). This sequence of events continues in the positive feedback of the depolarizing phase until some of the Na+ channels have become inactivated and enough of the potassium channels have opened to change the balance of current and result in a net outward current that produces membrane repolarization ( Fig. 14-7 ). After one action potential, the concentrations of Na+ and K+ have changed very little. The very small amount of Na+ entering and K+ leaving the cell as a result of this process is restored by the Na+ /K+ pump.[10]
Depolarizations too weak to activate enough Na+ channels to produce a net inward current are below the membrane's excitability threshold. The precise value of the threshold varies among different regions of the cell and also changes with activity. Directly after an impulse, when some Na+ channels are still inactivated and some K+ channels are still activated, the threshold is above its resting value and the membrane is "refractory" to stimulation. In the immediately repolarized membrane, as Na+ inactivation decays and K+ channels return to their closed conformation, the original threshold value is progressively restored.
The impulse is a wave of depolarization that is propagated along the axon by continuous coupling between excited and nonexcited regions of the membrane. Ionic current (the action current) entering the axon in the excited, depolarized region flows down the axoplasm and exits through the surrounding membrane, thus passively depolarizing the adjacent region (see Fig. 14-3 ). Although this local circuit current spreads away from the excited zone in both directions, the region behind the impulse, having just been depolarized, is absolutely refractory, and impulse propagation is thus unidirectional.
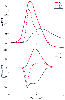 Figure 14-6
The membrane potential (Em
) and the voltage-gated
ionic conductance of sodium (gNa
) and potassium (gK
) that determine
the corresponding membrane currents (INa
and IK
) during a propagated
action potential. Modeled from the original studies of Hodgkin and Huxley on the
squid giant axon (see Hodgkin[8]
), these relationships
hold for almost all invertebrate and vertebrate nerve fibers. The direction of the
total ionic current (Ii
), which is the sum of INa
) and IK
,
is inward (negative values) for the depolarizing phase of the action potential and
outward (positive values) for the repolarizing phase.
Figure 14-6
The membrane potential (Em
) and the voltage-gated
ionic conductance of sodium (gNa
) and potassium (gK
) that determine
the corresponding membrane currents (INa
and IK
) during a propagated
action potential. Modeled from the original studies of Hodgkin and Huxley on the
squid giant axon (see Hodgkin[8]
), these relationships
hold for almost all invertebrate and vertebrate nerve fibers. The direction of the
total ionic current (Ii
), which is the sum of INa
) and IK
,
is inward (negative values) for the depolarizing phase of the action potential and
outward (positive values) for the repolarizing phase.
The local circuit current spreads rapidly along a length of insulated internode in a myelinated axon ( Fig. 14-3 ), and many nodes of Ranvier in sequence are depolarized to threshold with little intervening delay. Single impulses do not jump from node to node as separate, discrete events, but instead, active depolarization occurs simultaneously along several centimeters of the largest axons[11] (see Fig. 14-10 ). Indeed, the local circuit current is so robust that it can skip past two completely nonexcitable nodes and successfully stimulate a third.[12] If nodal excitability is partially reduced, for example, by inhibition of some of the Na+ channels, the amplitude of impulses in successive nodes falls decrementally, a process that can continue for many centimeters.[13] This situation probably occurs during certain phases of local anesthesia, as discussed later. However, when enough of the Na+ channels are blocked,
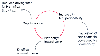 Figure 14-7
The action potential can be understood in terms of the
cyclic relationships between factors contributing to the regenerative, depolarizing
phase and the passive repolarizing phase. Positive factors (red
arrows) increase the rate of depolarization in a "positive-feedback loop,"
with each element in the cycle favoring the subsequent one. Negative factors (gray
arrows) decrease the depolarization rate by reducing or opposing the related
positive factor, with K+
efflux eventually dominating the ionic flow and
repolarizing the membrane.
Figure 14-7
The action potential can be understood in terms of the
cyclic relationships between factors contributing to the regenerative, depolarizing
phase and the passive repolarizing phase. Positive factors (red
arrows) increase the rate of depolarization in a "positive-feedback loop,"
with each element in the cycle favoring the subsequent one. Negative factors (gray
arrows) decrease the depolarization rate by reducing or opposing the related
positive factor, with K+
efflux eventually dominating the ionic flow and
repolarizing the membrane.
|
|
|
|
|
|
|
|
|
|
|
|
|