Left Ventricular-Arterial Coupling and Mechanical
Efficiency
Optimal transfer of stroke volume from the LV to the arterial
circulation requires appropriate matching of these mechanical systems. LV-arterial
coupling has most often been described using a series elastic chamber model of the
cardiovascular system ( Fig. 7-8
).
[128]
The elastance of the contracting LV (Ees
)
and the arterial vasculature (Ea
) is determined from LV end-systolic pressure-volume
and
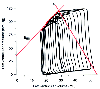 Figure 7-8
Left ventricular (LV) pressure versus LV volume diagrams
during inferior vena caval occlusion and the definition of LV-arterial coupling.
The LV maximal elastances of each pressure-volume diagram are used to calculate
the slope (EES
) of the end-systolic pressure-volume relationship. Effective
arterial elastance (EA
) is determined as the ratio of end-systolic arterial
pressure and stoke volume during steady-state hemodynamic conditions. In the pressure-volume
plane, EA
represents the magnitude of the slope connecting end-systole
to end-diastole. (Adapted from Hettrick DA, Pagel PS, Warltier DC: Desflurane,
sevoflurane, and isoflurane impair canine left ventricular-arterial coupling and
mechanical efficiency. Anesthesiology 85:403–413, 1996.)
Figure 7-8
Left ventricular (LV) pressure versus LV volume diagrams
during inferior vena caval occlusion and the definition of LV-arterial coupling.
The LV maximal elastances of each pressure-volume diagram are used to calculate
the slope (EES
) of the end-systolic pressure-volume relationship. Effective
arterial elastance (EA
) is determined as the ratio of end-systolic arterial
pressure and stoke volume during steady-state hemodynamic conditions. In the pressure-volume
plane, EA
represents the magnitude of the slope connecting end-systole
to end-diastole. (Adapted from Hettrick DA, Pagel PS, Warltier DC: Desflurane,
sevoflurane, and isoflurane impair canine left ventricular-arterial coupling and
mechanical efficiency. Anesthesiology 85:403–413, 1996.)
end-systolic arterial pressure-stroke volume relationships, respectively.[128]
[129]
[130]
The
ratio of Ees
to Ea
defines coupling between the LV and the
arterial circulation[128]
[129]
and provides a useful technique for assessment of the actions of drug, including
volatile anesthetics, on LV-arterial matching in vivo.[131]
[132]
Analysis of the pressure-volume relationship
also creates a framework for the study of LV mechanical efficiency defined by the
ratio of stroke work (SW) to the pressure-volume area (PVA).[133]
The influence of volatile anesthetics on LV-arterial coupling
and mechanical efficiency has been studied in the normal canine cardiovascular system
but has not been described in models of heart failure. LV-arterial coupling may
theoretically be maintained during anesthesia because declines in LV afterload may
balance simultaneous reductions in myocardial contractility. Low concentrations
of halothane (1 MAC), but not isoflurane, reduced Ees
/Ea
in
barbiturate-anesthetized, acutely instrumented dogs, consistent with depression of
mechanical coupling between the LV and arterial circulation.[134]
However, isoflurane also decreased Ees
/Ea
at 2 MAC, suggesting
that the vasodilating effects of this anesthetic were unable to compensate for the
relatively greater declines in contractility. Desflurane, sevoflurane, and isoflurane
maintained optimal LV-arterial coupling and mechanical efficiency as evaluated by
Ees
/Ea
and SW/PVA at low anesthetic concentrations (0.9 MAC)
by producing simultaneous declines in myocardial contractility and LV afterload ( Fig.
7-9
).[41]
However, mechanical matching
between the LV and
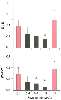 Figure 7-9
Histograms depicting left ventricular-arterial coupling
(Ees
/Ea
, top panel) and mechanical
efficiency (SW/PVA; bottom panel) before (control
1 [C1]); during 0.6, 0.9, and 1.2 minimum alveolar concentrations (MAC); and after
sevoflurane (control 2 [C2]). a, Significantly (P
< .05) different from C1; b, significantly (P
< .05) different from 0.9 MAC of sevoflurane; c, significantly (P
< .05) different from 1.2 MAC of sevoflurane. (Adapted from Hettrick
DA, Pagel PS, Warltier DC: Desflurane, sevoflurane, and isoflurane impair canine
left ventricular-arterial coupling and mechanical efficiency. Anesthesiology 85:403–413,
1996.)
Figure 7-9
Histograms depicting left ventricular-arterial coupling
(Ees
/Ea
, top panel) and mechanical
efficiency (SW/PVA; bottom panel) before (control
1 [C1]); during 0.6, 0.9, and 1.2 minimum alveolar concentrations (MAC); and after
sevoflurane (control 2 [C2]). a, Significantly (P
< .05) different from C1; b, significantly (P
< .05) different from 0.9 MAC of sevoflurane; c, significantly (P
< .05) different from 1.2 MAC of sevoflurane. (Adapted from Hettrick
DA, Pagel PS, Warltier DC: Desflurane, sevoflurane, and isoflurane impair canine
left ventricular-arterial coupling and mechanical efficiency. Anesthesiology 85:403–413,
1996.)
arterial vasculature and efficiency of total LV energy transfer to external stroke
work degenerates at higher anesthetic concentrations, indicating that anesthetic-induced
reductions in contractility are not appropriately balanced by declines in afterload.
Halothane (<1.0 MAC) has also been shown to reduce the ratio of oscillatory-to-mean
hydraulic power in vivo, indicating that this agent decreases LV mechanical efficiency
as well.[135]
Detrimental alterations in LV-arterial
coupling produced by volatile anesthetics contribute to reductions in overall cardiac
performance observed with these agents in vivo.
 Figure 7-8
Left ventricular (LV) pressure versus LV volume diagrams
during inferior vena caval occlusion and the definition of LV-arterial coupling.
The LV maximal elastances of each pressure-volume diagram are used to calculate
the slope (EES
) of the end-systolic pressure-volume relationship. Effective
arterial elastance (EA
) is determined as the ratio of end-systolic arterial
pressure and stoke volume during steady-state hemodynamic conditions. In the pressure-volume
plane, EA
represents the magnitude of the slope connecting end-systole
to end-diastole. (Adapted from Hettrick DA, Pagel PS, Warltier DC: Desflurane,
sevoflurane, and isoflurane impair canine left ventricular-arterial coupling and
mechanical efficiency. Anesthesiology 85:403–413, 1996.)
Figure 7-8
Left ventricular (LV) pressure versus LV volume diagrams
during inferior vena caval occlusion and the definition of LV-arterial coupling.
The LV maximal elastances of each pressure-volume diagram are used to calculate
the slope (EES
) of the end-systolic pressure-volume relationship. Effective
arterial elastance (EA
) is determined as the ratio of end-systolic arterial
pressure and stoke volume during steady-state hemodynamic conditions. In the pressure-volume
plane, EA
represents the magnitude of the slope connecting end-systole
to end-diastole. (Adapted from Hettrick DA, Pagel PS, Warltier DC: Desflurane,
sevoflurane, and isoflurane impair canine left ventricular-arterial coupling and
mechanical efficiency. Anesthesiology 85:403–413, 1996.)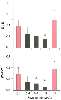 Figure 7-9
Histograms depicting left ventricular-arterial coupling
(Ees
/Ea
, top panel) and mechanical
efficiency (SW/PVA; bottom panel) before (control
1 [C1]); during 0.6, 0.9, and 1.2 minimum alveolar concentrations (MAC); and after
sevoflurane (control 2 [C2]). a, Significantly (P
< .05) different from C1; b, significantly (P
< .05) different from 0.9 MAC of sevoflurane; c, significantly (P
< .05) different from 1.2 MAC of sevoflurane. (Adapted from Hettrick
DA, Pagel PS, Warltier DC: Desflurane, sevoflurane, and isoflurane impair canine
left ventricular-arterial coupling and mechanical efficiency. Anesthesiology 85:403–413,
1996.)
Figure 7-9
Histograms depicting left ventricular-arterial coupling
(Ees
/Ea
, top panel) and mechanical
efficiency (SW/PVA; bottom panel) before (control
1 [C1]); during 0.6, 0.9, and 1.2 minimum alveolar concentrations (MAC); and after
sevoflurane (control 2 [C2]). a, Significantly (P
< .05) different from C1; b, significantly (P
< .05) different from 0.9 MAC of sevoflurane; c, significantly (P
< .05) different from 1.2 MAC of sevoflurane. (Adapted from Hettrick
DA, Pagel PS, Warltier DC: Desflurane, sevoflurane, and isoflurane impair canine
left ventricular-arterial coupling and mechanical efficiency. Anesthesiology 85:403–413,
1996.)