Left Ventricular Afterload
Although a definition of LV afterload that describes the mechanical
properties of the arterial vasculature opposing LV ejection is intuitively clear,
[136]
quantitative evaluation of afterload in vivo
remains difficult. Systemic vascular resistance, calculated as the ratio of mean
arterial pressure to cardiac output, is the most commonly used estimate of LV afterload.
However, systemic vascular resistance inadequately describes LV afterload[137]
because this index ignores the mechanical characteristics of the blood and arterial
walls, fails to account for the frequency-dependent,
phasic nature of arterial blood pressure and blood flow, and does not consider the
potential effects of arterial wave reflection. As a result, systemic vascular resistance
cannot be used reliably to quantify changes in LV afterload produced by drugs, including
volatile anesthetics, or by cardiovascular disease.[138]
Aortic input impedance [Zin
(ω)] obtained from power spectral or
Fourier series analysis of aortic pressure and blood flow waveforms provides a comprehensive
description of LV afterload because Zin
(ω) incorporates arterial
viscoelasticity, frequency dependence, and wave reflection. However, because analysis
of Zin
(ω) is conducted in the frequency domain and not as a function
of time, Zin
(ω) is most often interpreted using an electrical three-element
Windkessel model of the arterial circulation that describes characteristic aortic
impedance (Zc
), total arterial compliance (C), and total arterial resistance
(R). Zc
represents aortic resistance to LV ejection, C is determined
primarily by the compliance of the aorta and represents the energy storage component
of the arterial circulation, and R equals the combined resistances of the remaining
arterial vasculature ( Fig. 7-10
).
The three-element Windkessel model has been shown to approximate Zin
(ω)
closely under a variety of physiologic conditions.[139]
[140]
Volatile anesthetics alter Zin
(ω) by affecting
the mechanical properties of the arterial vascular tree.[141]
[142]
[143]
[144]
Isoflurane produced dose-related decreases in R in chronically instrumented dogs
consistent with the known effects of this drug on systemic vascular resistance, in
contrast to the results obtained with halothane.[143]
Isoflurane and halothane also caused similar increases in C and Zc
concomitant
with reductions in mean arterial pressure. The major difference between the effects
of isoflurane and halothane on LV afterload derived from the Windkessel model of
Zin
(ω) was related to R, a property of arteriolar resistance vessels,
and not to C or Zc
, the mechanical characteristics of the aorta ( Fig.
7-11
). A subsequent investigation demonstrated that desflurane, but not
sevoflurane, also reduced R in dogs.[144]
In contrast
to
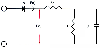 Figure 7-10
Schematic diagram depicting the three-element Windkessel
model of the arterial circulation. Diode A represents the aortic valve. Time-dependent
blood flow [F(t)] and blood pressure [P(t)] entering the arterial system first encounters
the resistance of the ascending aorta (characteristic aortic impedance [Zc]). Further
flow is dictated by total arterial resistance (R) and total arterial compliance (C),
the energy storage component of the arterial vasculature. (Adapted from
Hettrick DA, Pagel PS, Warltier DC: Differential effects of isoflurane and halothane
on aortic input impedance quantified using a three-element Windkessel model. Anesthesiology
83:361–373, 1995.)
Figure 7-10
Schematic diagram depicting the three-element Windkessel
model of the arterial circulation. Diode A represents the aortic valve. Time-dependent
blood flow [F(t)] and blood pressure [P(t)] entering the arterial system first encounters
the resistance of the ascending aorta (characteristic aortic impedance [Zc]). Further
flow is dictated by total arterial resistance (R) and total arterial compliance (C),
the energy storage component of the arterial vasculature. (Adapted from
Hettrick DA, Pagel PS, Warltier DC: Differential effects of isoflurane and halothane
on aortic input impedance quantified using a three-element Windkessel model. Anesthesiology
83:361–373, 1995.)
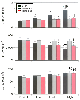 Figure 7-11
Histograms depict the effects of sodium nitroprusside
(SNP), halothane, and isoflurane on total arterial compliance (C, top),
total arterial resistance (R, middle), and characteristic
aortic impedance (ZC
, bottom). The low,
medium, and high doses of volatile anesthetics are 1.25, 1.5, and 1.75 minimum alveolar
concentrations (MACs), respectively. SNP doses produce comparable changes in mean
arterial pressure. *, Significantly (P <
.05) different from the control; †, significantly (P
< .05) different from the low dose; §, significantly (P
< .05) different from the middle dose; ‡, significantly (P
< .05) different from halothane. (Adapted from Hettrick DA, Pagel PS,
Warltier DC: Differential effects of isoflurane and halothane on aortic input impedance
quantified using a three-element Windkessel model. Anesthesiology 83:361–373,
1995.)
Figure 7-11
Histograms depict the effects of sodium nitroprusside
(SNP), halothane, and isoflurane on total arterial compliance (C, top),
total arterial resistance (R, middle), and characteristic
aortic impedance (ZC
, bottom). The low,
medium, and high doses of volatile anesthetics are 1.25, 1.5, and 1.75 minimum alveolar
concentrations (MACs), respectively. SNP doses produce comparable changes in mean
arterial pressure. *, Significantly (P <
.05) different from the control; †, significantly (P
< .05) different from the low dose; §, significantly (P
< .05) different from the middle dose; ‡, significantly (P
< .05) different from halothane. (Adapted from Hettrick DA, Pagel PS,
Warltier DC: Differential effects of isoflurane and halothane on aortic input impedance
quantified using a three-element Windkessel model. Anesthesiology 83:361–373,
1995.)
the findings for isoflurane, desflurane did not affect C and Zc
, suggesting
that this agent does not alter the mechanical properties of the aorta. The inverse
relationship between C and mean arterial pressure remains unchanged by volatile anesthetics,
[143]
[144]
unlike
the findings with the arterial vasodilator sodium nitroprusside[143]
[145]
or the intravenous anesthetic propofol.[146]
Isoflurane and halothane produce alterations in Zin
(ω)
in cardiomyopathic dogs that are substantially different from those observed in normal
dogs.[147]
These volatile anesthetics decreased
arterial pressure but did not affect C and Zc
in the presence of LV dysfunction.
Halothane increased R and isoflurane did not reduce R in dogs with dilated cardiomyopathy.
Neither isoflurane nor halothane reduce arterial hydraulic resistance or favorably
improve the rectifying properties of the aorta in dogs with pacing-induced cardiomyopathy
( Fig. 7-12
). The findings
suggest that volatile agents do not exert beneficial actions in LV afterload in the
presence of failing myocardium.
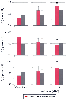 Figure 7-12
Histograms illustrate total arterial compliance (C, top
panel), total arterial resistance (R, middle panel),
and characteristic aortic impedance (ZC
, bottom panel)
in the conscious state and during 1.1 and 1.5 minimum alveolar concentrations (MACs)
of isoflurane in dogs before (red bars) and after
(gray bars) the development of pacing-induced cardiomyopathy.
a, Significantly (P < .05) different from normal
myocardium. (Adapted from Hettrick DA, Pagel PS, Kersten JR, et al: The
effects of isoflurane and halothane on left ventricular afterload in dogs with dilated
cardiomyopathy. Anesth Analg 85:979–986, 1997.)
Figure 7-12
Histograms illustrate total arterial compliance (C, top
panel), total arterial resistance (R, middle panel),
and characteristic aortic impedance (ZC
, bottom panel)
in the conscious state and during 1.1 and 1.5 minimum alveolar concentrations (MACs)
of isoflurane in dogs before (red bars) and after
(gray bars) the development of pacing-induced cardiomyopathy.
a, Significantly (P < .05) different from normal
myocardium. (Adapted from Hettrick DA, Pagel PS, Kersten JR, et al: The
effects of isoflurane and halothane on left ventricular afterload in dogs with dilated
cardiomyopathy. Anesth Analg 85:979–986, 1997.)
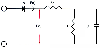 Figure 7-10
Schematic diagram depicting the three-element Windkessel
model of the arterial circulation. Diode A represents the aortic valve. Time-dependent
blood flow [F(t)] and blood pressure [P(t)] entering the arterial system first encounters
the resistance of the ascending aorta (characteristic aortic impedance [Zc]). Further
flow is dictated by total arterial resistance (R) and total arterial compliance (C),
the energy storage component of the arterial vasculature. (Adapted from
Hettrick DA, Pagel PS, Warltier DC: Differential effects of isoflurane and halothane
on aortic input impedance quantified using a three-element Windkessel model. Anesthesiology
83:361–373, 1995.)
Figure 7-10
Schematic diagram depicting the three-element Windkessel
model of the arterial circulation. Diode A represents the aortic valve. Time-dependent
blood flow [F(t)] and blood pressure [P(t)] entering the arterial system first encounters
the resistance of the ascending aorta (characteristic aortic impedance [Zc]). Further
flow is dictated by total arterial resistance (R) and total arterial compliance (C),
the energy storage component of the arterial vasculature. (Adapted from
Hettrick DA, Pagel PS, Warltier DC: Differential effects of isoflurane and halothane
on aortic input impedance quantified using a three-element Windkessel model. Anesthesiology
83:361–373, 1995.) Figure 7-11
Histograms depict the effects of sodium nitroprusside
(SNP), halothane, and isoflurane on total arterial compliance (C, top),
total arterial resistance (R, middle), and characteristic
aortic impedance (ZC
, bottom). The low,
medium, and high doses of volatile anesthetics are 1.25, 1.5, and 1.75 minimum alveolar
concentrations (MACs), respectively. SNP doses produce comparable changes in mean
arterial pressure. *, Significantly (P <
.05) different from the control; †, significantly (P
< .05) different from the low dose; §, significantly (P
< .05) different from the middle dose; ‡, significantly (P
< .05) different from halothane. (Adapted from Hettrick DA, Pagel PS,
Warltier DC: Differential effects of isoflurane and halothane on aortic input impedance
quantified using a three-element Windkessel model. Anesthesiology 83:361–373,
1995.)
Figure 7-11
Histograms depict the effects of sodium nitroprusside
(SNP), halothane, and isoflurane on total arterial compliance (C, top),
total arterial resistance (R, middle), and characteristic
aortic impedance (ZC
, bottom). The low,
medium, and high doses of volatile anesthetics are 1.25, 1.5, and 1.75 minimum alveolar
concentrations (MACs), respectively. SNP doses produce comparable changes in mean
arterial pressure. *, Significantly (P <
.05) different from the control; †, significantly (P
< .05) different from the low dose; §, significantly (P
< .05) different from the middle dose; ‡, significantly (P
< .05) different from halothane. (Adapted from Hettrick DA, Pagel PS,
Warltier DC: Differential effects of isoflurane and halothane on aortic input impedance
quantified using a three-element Windkessel model. Anesthesiology 83:361–373,
1995.) Figure 7-12
Histograms illustrate total arterial compliance (C, top
panel), total arterial resistance (R, middle panel),
and characteristic aortic impedance (ZC
, bottom panel)
in the conscious state and during 1.1 and 1.5 minimum alveolar concentrations (MACs)
of isoflurane in dogs before (red bars) and after
(gray bars) the development of pacing-induced cardiomyopathy.
a, Significantly (P < .05) different from normal
myocardium. (Adapted from Hettrick DA, Pagel PS, Kersten JR, et al: The
effects of isoflurane and halothane on left ventricular afterload in dogs with dilated
cardiomyopathy. Anesth Analg 85:979–986, 1997.)
Figure 7-12
Histograms illustrate total arterial compliance (C, top
panel), total arterial resistance (R, middle panel),
and characteristic aortic impedance (ZC
, bottom panel)
in the conscious state and during 1.1 and 1.5 minimum alveolar concentrations (MACs)
of isoflurane in dogs before (red bars) and after
(gray bars) the development of pacing-induced cardiomyopathy.
a, Significantly (P < .05) different from normal
myocardium. (Adapted from Hettrick DA, Pagel PS, Kersten JR, et al: The
effects of isoflurane and halothane on left ventricular afterload in dogs with dilated
cardiomyopathy. Anesth Analg 85:979–986, 1997.)