|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Separation of the two lungs during thoracic operations or procedures has several absolute and relative indications ( Table 49-10 ).
| Absolute |
| 1. Isolation of one lung from the other to avoid spillage or contamination |
| A. Infection |
| B. Massive hemorrhage |
| 2. Control of the distribution of ventilation |
| A. Bronchopleural fistula |
| B. Bronchopleural cutaneous fistula |
| C. Surgical opening of a major conducting airway |
| D. Giant unilateral lung cyst or bulla |
| E. Tracheobronchial tree disruption |
| F. Life-threatening hypoxemia from unilateral lung disease |
| 3. Unilateral bronchopulmonary lavage |
| A. Pulmonary alveolar proteinosis |
| Relative |
| 1. Surgical exposure—high priority |
| A. Thoracic aortic aneurysm |
| B. Pneumonectomy |
| C. Upper lobectomy |
| D. Mediastinal exposure |
| E. Thoracoscopy |
| 2. Surgical exposure—medium (lower) priority |
| A. Middle and lower lobectomies and subsegmental resections |
| B. Esophageal resection |
| C. Procedures on the thoracic spine |
| 3. Post-cardiopulmonary bypass status after removal of totally occluding chronic unilateral pulmonary emboli |
| 4. Severe hypoxemia from unilateral lung disease |
Separation of the two lungs for any of the absolute indications discussed here should be considered a lifesaving maneuver because failure to separate the lungs under any of these conditions could result in a life-threatening complication or situation. There are three general absolute indications for separating the lungs (see Table 49-10 ). First, separation of one lung from the other is absolutely necessary to prevent spillage of pus or blood from an infected (abscessed) lung or bleeding lung, respectively, to a noninvolved lung. Acute contamination of a lung with either blood or pus from the other lung usually results in severe massive (bilateral) atelectasis, pneumonia, and sepsis. Second, a number of unilateral lung problems can prevent adequate ventilation of the noninvolved side. A large bronchopleural or bronchopleural-cutaneous fistula or a surgically opened conducting airway has such low resistance to gas flow that a tidal inspiration delivered by positive pressure will exit through the low-resistance pathway, and it may become impossible to ventilate the other, more normal lung adequately. A giant unilateral bulla or cyst may rupture if exposed to positive-pressure ventilation and result in a tension pneumothorax or pneumomediastinum. Very severe or life-threatening hypoxemia as a result of unilateral lung disease may require differential lung ventilation and PEEP.[239] Finally, positive-pressure ventilation of a lung with disruption of the tracheobronchial tree can result in dissection of gas into the pulmonary interstitial space or mediastinum and cause a tension pneumomediastinum. Third, separation of the lungs is absolutely necessary to perform unilateral bronchopulmonary lavage in patients with pulmonary alveolar proteinosis (and rarely, asthma or cystic fibrosis).
Separation of the lungs has a large number of relative indications, and they are all for the purpose of facilitating surgical exposure by collapsing the lung in the operative hemithorax. These relative indications can be divided into high-priority and low-priority categories (see Table 49-10 ). Of the relative indications, repair of a thoracic aortic aneurysm usually has the highest priority because it may require exposure of the thoracic aorta as it runs the entire length of the left hemithorax. Pneumonectomy, especially if performed through a median sternotomy,[240] is greatly aided by the wide exposure of the lung hilum that is afforded by collapse of the operative lung. Similarly, upper lobectomy, which is technically the most difficult lobectomy, and many mediastinal exposures may be made much easier by eliminating ventilation to the lung on the side of the procedure. Examination of the pleural space (thoracoscopy) and pulmonary resection through a thoracoscope are considerably aided by collapse of the ipsilateral lung. The surgical items in the medium-priority category do not routinely require collapse of the lung on the operative side, but they still significantly aid surgical exposure and eliminate the need for the surgeon to handle (retract, compress, pack away) the operative lung. Severe intraoperative retraction of the lung on the operated side can traumatize the operative lung and impair gas exchange both intraoperatively[241] and postoperatively.[38] [39] The lower-priority items consist of middle and lower
In general, three types of devices are available for providing one-lung ventilation during anesthesia: DLTs, bronchial blockers,[242] and endobronchial tubes. DLTs have come to be considered the lung separation technique of choice for most thoracic surgery and are discussed later in detail. Bronchial blockers are becoming increasingly sophisticated and range from the long-used Fogarty vascular embolectomy catheter,[243] to the more recently developed Torque Control Blocker Univent (Vitaid, Lewiston, NY),[244] to the more recent wire-guided endobronchial blocker (Arndt blocker; Cook Critical Care, Bloomington, IN),[245] and they are also described at length later. Endobronchial tubes are not often used for lung separation today and are only briefly described at the end of the chapter. The primary reason that DLTs are favored over bronchial blockers and endobronchial tubes for lung separation is that they are more versatile than the other two devices. The most important DLT function not available with a bronchial blocker is the ability to suction secretions blindly as well
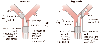 Figure 49-14
Schematic diagram depicting the essential features and
parts of left-sided and right-sided double-lumen endotracheal tubes. LUL, left upper
lobe; RUL, right upper lobe. (From Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-14
Schematic diagram depicting the essential features and
parts of left-sided and right-sided double-lumen endotracheal tubes. LUL, left upper
lobe; RUL, right upper lobe. (From Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
When compared with a bronchial blocker, the use of a DLT has two firm disadvantages (contraindications). First, very distorted tracheobronchial tree anatomy, including exophytic and stenotic lesions, as well as tortuosity, may preclude successful correct placement or positioning of a DLT. Second, changing from a DLT to a single-lumen tube during or at the end of an operation can be expected to be a difficult or risky procedure (or both) on occasion. Such a situation might occur in a patient with a relatively difficult airway before surgery who undergoes a long operation requiring considerable intravenous fluids; one would expect the airway to be edematous and thus a postoperative tube change to be more hazardous in that setting.
DLTs have two relatively minor disadvantages, both related to the fact that the lumens of a DLT may be narrow. First, suctioning may be more difficult down a narrow lumen, but this is not usually a problem with the new disposable Robertshaw type of DLTs, which have nonadhering suction catheters that slide easily down the lumens. Second, although airway resistance may be increased with a narrow lumen, the increased resistance can be easily overcome by positive-pressure ventilation. [246]
A DLT is essentially two catheters bonded together side by side, with each lumen intended to ventilate one of the two lungs. DLTs are made as left- and right-sided tubes. With a left-sided tube, the left lung catheter is placed into the left main stem bronchus, whereas the right lung catheter ends in the trachea; therefore, for a left-sided tube, the left lung catheter is longer than the right lung catheter ( Fig. 49-14 ). With a right-sided tube, the right lung catheter is placed into the right main stem bronchus,
The DLTs that are now used for lung separation and one-lung ventilation are the Carlens and the Robertshaw. The Robertshaw type of tube is by far the more commonly used, and the disposable polyvinyl chloride (PVC) Robertshaw tube has significantly replaced the red rubber Robertshaw tube (the former is easier to pass, can be positioned more quickly, and causes less mucosal damage).[247] Consequently, the modern PVC tube will be described in great detail.
The left-sided Carlens tube ( Fig. 49-15 ) was the first DLT used for one-lung ventilation.[248] The tube had a carinal hook to aid in proper placement and minimize tube advancement after placement. Potential problems with carinal hooks include increased difficulty (more rotations) and laryngeal trauma during intubation, amputation of the hook during or after passage, malpositioning of the tube as a result of the hook, and physical interference during pneumonectomy.[249]
The original Robertshaw DLT, introduced in 1962, was made as a reusable red rubber tube ( Fig. 49-16 ). [250] This
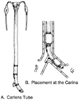 Figure 49-15
A, Sketch of the red rubber
(nondisposable) Carlens double-lumen endotracheal tube. B,
Close-up of placement of the red rubber Carlens double-lumen endotracheal tube at
the carina. Note that the left endobronchial lumen and carinal hook straddle the
carina. (From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia,
WB Saunders, 1987.)
Figure 49-15
A, Sketch of the red rubber
(nondisposable) Carlens double-lumen endotracheal tube. B,
Close-up of placement of the red rubber Carlens double-lumen endotracheal tube at
the carina. Note that the left endobronchial lumen and carinal hook straddle the
carina. (From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia,
WB Saunders, 1987.)
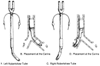 Figure 49-16
A, Sketch of the left-sided
red rubber Robertshaw double-lumen endotracheal tube. B,
Close-up of placement of the left-sided Robertshaw double-lumen endotracheal tube
at the carina. C, Sketch of the right-sided Robertshaw
double-lumen endotracheal tube. D, Close-up of placement
of the right-sided Robertshaw double-lumen endotracheal tube at the carina. (From
Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-16
A, Sketch of the left-sided
red rubber Robertshaw double-lumen endotracheal tube. B,
Close-up of placement of the left-sided Robertshaw double-lumen endotracheal tube
at the carina. C, Sketch of the right-sided Robertshaw
double-lumen endotracheal tube. D, Close-up of placement
of the right-sided Robertshaw double-lumen endotracheal tube at the carina. (From
Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
The first plastic Robertshaw DLTs were made by National Catheter Corporation, which has since become part of Mallinckrodt. Robertshaw DLTs are now manufactured by Mallinckrodt, Rusch, Portex, and Sheridan. The Robertshaw type of tube is presently made of a clear nontoxic tissue-implantable plastic (denoted by the marking Z-79) and is disposable (see Fig. 49-14 ). The tubes are made in sizes 41, 39, 37, 35, 28, and 26 French (the internal diameter of each lumen is approximately 6.5, 6.0, 5.5, 5.0, 4.5, and 4.0 mm, respectively). The 26 and 28 French tubes are available only as left-sided models. These tubes are relatively easy to insert and have appropriate end-of-lumen and cuff arrangements that minimize lobar obstruction. The endobronchial cuff is brilliant blue, which is an important recognition feature when using a fiberoptic bronchoscope. The
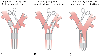 Figure 49-17
Use of left-sided and right-sided double-lumen endotracheal
tubes for left and right lung surgery (as indicated by the clamp). A,
When surgery is performed on the right lung, a left-sided double-lumen endotracheal
tube should be used. B, When surgery is performed
on the left lung, a right-sided double-lumen endotracheal tube can be used. However,
because of uncertainty about alignment of the right upper lobe ventilation slot with
the right upper lobe orifice, a left-sided double-lumen endotracheal tube can also
be used for left lung surgery. C, If the left lung
surgery requires a clamp to be placed high on the left main stem bronchus, the left
endobronchial cuff should be deflated, the left-sided double-lumen endotracheal tube
pulled back into the trachea, and the right lung ventilated through both lumens (use
the double-lumen endotracheal tube as a single-lumen tube). (From Benumof
JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-17
Use of left-sided and right-sided double-lumen endotracheal
tubes for left and right lung surgery (as indicated by the clamp). A,
When surgery is performed on the right lung, a left-sided double-lumen endotracheal
tube should be used. B, When surgery is performed
on the left lung, a right-sided double-lumen endotracheal tube can be used. However,
because of uncertainty about alignment of the right upper lobe ventilation slot with
the right upper lobe orifice, a left-sided double-lumen endotracheal tube can also
be used for left lung surgery. C, If the left lung
surgery requires a clamp to be placed high on the left main stem bronchus, the left
endobronchial cuff should be deflated, the left-sided double-lumen endotracheal tube
pulled back into the trachea, and the right lung ventilated through both lumens (use
the double-lumen endotracheal tube as a single-lumen tube). (From Benumof
JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
A left-sided DLT should be used for right thoracotomies requiring collapse of the right lung and ventilation of the left lung ( Fig. 49-17 ). A left-or right-sided tube may be used for left thoracotomies requiring collapse of the left lung and ventilation of the right lung (see Fig. 49-17 ). However, because the right upper lobe ventilation slot of a right-sided tube has to be closely apposed to the right upper lobe orifice to allow unobstructed right upper lobe ventilation and because of the considerable anatomic variation in the exact position of the right upper lobe orifice and therefore in the length
In general, as height and weight increase, the appropriate DLT size increases, although height correlates much better than weight.[252] Accordingly, the largest size DLT that fits should be used to minimize airway resistance and increase the ease of passage of the fiberoptic bronchoscope and suction catheter. Short patients (4'6" to 5'5") should receive a 35 to 37 French left-sided DLT; for medium-height patients (5'5" to 5'10"), a 37–39 French left-sided DLT is recommended; and for tall patients (5'11" to 6'4"), a 39 to 41 French left-sided DLT is optimum. [252] In our experience, airways tend to be larger than would be predicted by height alone in chronic smokers and in patients with bronchiectasis or chronic pulmonary infections (e.g., cystic fibrosis). These patients can often tolerate larger DLTs than would be predicted by their height. Additionally, men tend to have slightly larger airways than women of same height do.
Young teenagers (13 to 14 years old) can frequently use an adult-sized 35 French DLT. The smallest left-sided DLTs made by Mallinckrodt are 32, 28, and 26 French; they can be used by 12-, 10-, and 8-year-old children, respectively. The smallest right-sided tube is the 32 French; the 28 and 26 French tubes are available only as left-sided DLTs. Leyland Rubber has made some special-order right-and left-sided DLTs for 6- to 8-year-old children, largely for bronchopulmonary lavage of alveolar proteinosis. For smaller children, bronchial blockers (see later) and main stem intubation are techniques typically used when lung separation is necessary. There are 3.5 and 4.5 Univent tubes (Fuji systems) available for 8- to 10-year-old children. Additionally, Marraro bilumen uncuffed tubes have been used in neonates weighing as little as 1500 g and in 5-year-olds.[253]
Before intubation with a DLT or induction of anesthesia, the patient should have a complete airway examination (see Chapter 25 and Chapter 42 ). If the trachea appears difficult to intubate, the patient should be intubated awake and either a Univent tube or a conventional endotracheal tube inserted and a bronchial blocker used for lung separation (see later). However, if the patient's airway examination does not provoke concern regarding difficult intubation, general anesthetic induction and the following "conventional intubation sequence" may be followed.
Before intubation with a DLT, both cuffs and the lumen connections are examined for function. A 3- to 5-mL syringe should be placed on the end of the bronchial cuff pilot tube, and a 10-mL syringe with stopcock should also be placed on the tracheal cuff pilot tube. Because the high-volume, low-pressure cuffs can easily be torn by teeth, the distal tube is coated with a lubricating ointment, preferably containing a local anesthetic, and a thin mouth guard can be placed over the patient's upper incisors to minimize this possibility. If a less than optimal view of the larynx is anticipated, the stylet that is packaged with the tube is lubricated, inserted into the left lumen, and appropriately curved. The patient is then anesthetized and paralyzed as described previously. A curved open-phalange blade (e.g., MacIntosh) is usually preferred for laryngoscopy because it approximates the curvature of the tube and therefore provides the largest possible area through which to pass the tube. However, a straight (Miller) blade may be a better choice in patients with overriding upper teeth or an excessively anterior larynx.
The Robertshaw-type DLT is passed with the distal curvature initially concave anteriorly ( Fig. 49-18A ). After the tube tip passes the larynx and while anterior force on the laryngoscope is continued, the stylet (if used) is removed and the tube is carefully rotated 90 degrees (so that the distal curve is now concave toward the appropriate side and the proximal curve is concave anteriorly) to allow endobronchial intubation on the appropriate side ( Fig. 49-18B ). Continued anterior force by the laryngoscope during tube rotation prevents the hypopharyngeal structures from falling in around the tube and interfering with free 90-degree rotation of the distal tube tip. Failure to obtain a near 90-degree rotation of the distal tube tip while the proximal end rotates 90 degrees will cause either a kink or a twist in the shaft of the tube and/or prevent the distal end of the lumen from lying free in the main stem bronchus (i.e., not up against the bronchial wall). After rotation, the tube is advanced until most of it is inserted ( Fig. 49-18C ).[252] When the proper depth of insertion has been achieved (defined as when the cephalad surface of the bronchial cuff is immediately below the carinal bifurcation), the average depth of insertion for both male and female patients 170 cm tall is 29 cm, and for each 10-cm increase or decrease in height, the average placement depth is increased or decreased by 1 cm.[252] Correlation between the depth of insertion and height is highly significant (P < .0001) for both male and female patients. Nevertheless, it should be understood that the depth of DLT insertion at any given height is still normally distributed, and correct DLT position should always be confirmed fiberoptically after initial placement. DLTs may also be passed successfully by means of a tracheostomy, although it should be remembered that the tracheal cuff may be at the tracheal stoma or lie partly outside the trachea in this situation.[254] Therefore, one may prefer to use a bronchial blocker[242] or a specially manufactured (i.e., short) nondisposable DLT for these particular patients. [255]
Once the tube tip is thought to be in an endobronchial position, the following checklist is used to ensure proper
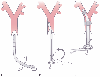 Figure 49-18
Schematic diagram depicting passage of the left-sided
double-lumen endotracheal tube in a supine patient. A,
The tube is held with the distal curvature concave anteriorly and the proximal curve
concave to the right and in a plane parallel to the floor. The tube is then inserted
through the vocal cords until the bronchial cuff passes the vocal cords. The stylet
is then removed. B, The tube is rotated 90 degrees
counterclockwise so that the distal curvature is concave anteriorly and the proximal
curvature is concave to the left and in a plane parallel to the floor. C,
The tube is inserted until either mild resistance to further passage is encountered
or the end of the common molding of the two lumens is at the teeth. Both cuffs are
then inflated, and both lungs are ventilated. Finally, one side is clamped while
the other side is ventilated and vice versa. (See the text for further explanation.)
(From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders,
1987.)
Figure 49-18
Schematic diagram depicting passage of the left-sided
double-lumen endotracheal tube in a supine patient. A,
The tube is held with the distal curvature concave anteriorly and the proximal curve
concave to the right and in a plane parallel to the floor. The tube is then inserted
through the vocal cords until the bronchial cuff passes the vocal cords. The stylet
is then removed. B, The tube is rotated 90 degrees
counterclockwise so that the distal curvature is concave anteriorly and the proximal
curvature is concave to the left and in a plane parallel to the floor. C,
The tube is inserted until either mild resistance to further passage is encountered
or the end of the common molding of the two lumens is at the teeth. Both cuffs are
then inflated, and both lungs are ventilated. Finally, one side is clamped while
the other side is ventilated and vice versa. (See the text for further explanation.)
(From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders,
1987.)
In summary, when DLT position is correct, the breath sounds are normal and follow the expected unilateral pattern with unilateral clamping, the chest rises and falls in accordance with the breath sounds, the ventilated lung feels reasonably compliant, no leaks are present, and respiratory gas moisture appears and disappears with each tidal ventilation. Conversely, when the DLT is malpositioned, any or all of the following may occur: breath sounds may be poor and correlate poorly with unilateral clamping, chest movements may not follow the expected pattern, the ventilated lung may feel noncompliant, leaks may be present, or the respiratory gas moisture in the clear tubing may be relatively stationary. It is very
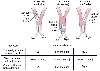 Figure 49-19
Three major malpositions (involving a whole lung) of
a left-sided double-lumen endotracheal tube can occur. The tube can be in too far
on the left (both lumens are in the left main stem bronchus), out too far (both lumens
are in the trachea), or down the right main stem bronchus (at least the left lumen
is in the right main stem bronchus). In each of these three malpositions, the left
cuff, when fully inflated, can completely block the right lumen. Inflation and deflation
of the left cuff while the left lumen is clamped create a breath sound differential
diagnosis of tube malposition. (See the text for a full explanation.) L, left;
R, right; ↓, decreased. (From Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-19
Three major malpositions (involving a whole lung) of
a left-sided double-lumen endotracheal tube can occur. The tube can be in too far
on the left (both lumens are in the left main stem bronchus), out too far (both lumens
are in the trachea), or down the right main stem bronchus (at least the left lumen
is in the right main stem bronchus). In each of these three malpositions, the left
cuff, when fully inflated, can completely block the right lumen. Inflation and deflation
of the left cuff while the left lumen is clamped create a breath sound differential
diagnosis of tube malposition. (See the text for a full explanation.) L, left;
R, right; ↓, decreased. (From Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
When it is believed, on the basis of clinical signs, that the DLT is malpositioned, it is theoretically possible to diagnose the malposition of the tube more precisely by a combination of several unilateral clamping, chest auscultation, and left endobronchial cuff inflation-deflation maneuvers ( Fig. 49-19 ). With reference to a left-sided DLT, there are three possible gross malpositions: in too far on the left (both lumens are in left main stem bronchus), out too far (both lumens are in the trachea), and in or down the right main stem bronchus (at least the left lumen is in the right main stem bronchus). When the right (tracheal) side is clamped and the tube is in too far on the left side, breath sounds are heard only on the left side. When the tube is out too far and the right side is clamped, breath sounds are heard bilaterally. When the tube is in or down the right side and the right side is clamped, breath sounds are heard only on the right side. When the left side is clamped and the left endobronchial cuff is inflated, the right lumen is blocked by the left cuff in all three malpositions. Consequently, with the left side clamped and the left cuff inflated, no or very diminished breath sounds are heard bilaterally in all three of the malpositions. When the left side is clamped and the left cuff is deflated so that the right lumen is no longer blocked by the left cuff, breath sounds are heard only on the left side when the tube is in too far on the left, bilaterally when the tube is out too far, and only on the right side when the tube is in the right side. The left cuff inflation and deflation findings provide the key diagnostic data because they essentially define the position of the right tracheal lumen by blocking and unblocking it with the left cuff.
In several situations, however, these unilateral clamping, auscultation, and cuff inflation and deflation maneuvers for determining the integrity of lung separation are either unreliable or impossible. First and most importantly, when the patient is in the LDP, has had a skin preparation, and is draped, access to the chest wall is impossible, and the anesthesiologist cannot listen to the chest. Second, the presence of unilateral or bilateral lung disease, either preexisting before anesthesia and surgery or induced by anesthesia, may markedly obscure the crispness of the chest auscultation end points. Third, the diagnosis of exactly where the DLT is located may be confused when the tube is just slightly malpositioned. Fourth, the tube may have moved as a result of some event, such as coughing, head flexion or extension while turning into the LDP, or tracheal manipulation and hilar retraction by the surgeon. Finally, some combination of these factors may culminate in uncertainty about where the DLT has located. The solution to any uncertainty about the exact position of the DLT is to determine the position by fiberoptic bronchoscopy.
As noted, even when a DLT is thought to be in proper position on the basis of clinical signs, subsequent fiberoptic bronchoscopy will reveal an incidence of malpositioning as high as 78%.[256] Indeed, when the position of the DLT is checked only by clinical signs, in up to 25% of cases there may be intraoperative problems with either deflating the nondependent lung, ventilating the dependent lung, or completely separating the two lungs.[256] [258] Given the frequent incidence of malpositioned DLTs when DLT position is determined by only auscultation (i.e., "blindly") and the potentially serious consequences associated with a malpositioned DLT, it is only a matter of simple common sense to routinely use a fiberoptic bronchoscope to easily, quickly, and precisely determine the position of the DLT.
The exact position of a left-sided DLT can be ascertained at any time, in less than a minute, by simply passing a
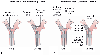 Figure 49-20
This schematic diagram depicts the complete fiberoptic
bronchoscopy picture of left-sided double-lumen endotracheal tubes (both the desired
view and the view to be avoided from both of the lumens). A,
When the bronchoscope is passed down the left lumen of a left-sided tube, the endoscopist
should see a very slight left luminal narrowing and a clear straight-ahead view of
the bronchial carina off in the distance. Excessive left luminal narrowing should
be avoided. B, When the bronchoscope is passed down
the right lumen of a left-sided tube, the endoscopist should see a clear straight-ahead
view of the tracheal carina and the upper surface of the blue left endobronchial
cuff just below the tracheal carina. Excessive pressure in the endobronchial cuff,
as manifested by tracheal carinal deviation to the right and herniation of the endobronchial
cuff over the carina, should be avoided. (From Benumof JL: Anesthesia for
Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-20
This schematic diagram depicts the complete fiberoptic
bronchoscopy picture of left-sided double-lumen endotracheal tubes (both the desired
view and the view to be avoided from both of the lumens). A,
When the bronchoscope is passed down the left lumen of a left-sided tube, the endoscopist
should see a very slight left luminal narrowing and a clear straight-ahead view of
the bronchial carina off in the distance. Excessive left luminal narrowing should
be avoided. B, When the bronchoscope is passed down
the right lumen of a left-sided tube, the endoscopist should see a clear straight-ahead
view of the tracheal carina and the upper surface of the blue left endobronchial
cuff just below the tracheal carina. Excessive pressure in the endobronchial cuff,
as manifested by tracheal carinal deviation to the right and herniation of the endobronchial
cuff over the carina, should be avoided. (From Benumof JL: Anesthesia for
Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
With reference to a right-sided DLT, while looking down the left (tracheal) lumen, the endoscopist should see a clear straight-ahead view of the tracheal carina and the right lumen going off to the right ( Fig. 49-21A ). The upper surface of the right endobronchial balloon may not be visible below the tracheal carina. While looking down the right lumen, the endoscopist should see a very slight narrowing of the right lumen, as well as the right middle-lower lobe bronchial carina distal to the end of the tube. Most importantly, the endoscopist should locate the right upper lobe ventilation slot and be able to look directly into the right upper lobe orifice through the right upper lobe ventilation slot by simply flexing the tip of the fiberoptic bronchoscope superiorly and laterally ( Fig. 49-21B ). The right upper lobe ventilation slot should not override the bronchial mucosa, and the bronchial mucosa should not be covering any of the right upper lobe ventilation slot.
In our experience, in 85% of cases the clinical signs (breath sounds, chest movements, compliance of the lung or lungs, movement of respiratory gas moisture)
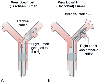 Figure 49-21
This schematic diagram portrays the use of a fiberoptic
bronchoscope to determine precise right-sided double-lumen tube position. A,
When the fiberoptic bronchoscope is passed down the left (tracheal) lumen, the endoscopist
should see a clear straight-ahead view of the tracheal carina and the right lumen
going off into the right main stem bronchus. B, When
the fiberoptic bronchoscope is passed down the right (bronchial) lumen, the endoscopist
should see the bronchial carina off in the distance; when the fiberoptic bronchoscope
is flexed cephalad and passed through the right upper lobe ventilation slot, the
right upper lobe bronchial orifice should be visualized. (From Benumof JL:
Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-21
This schematic diagram portrays the use of a fiberoptic
bronchoscope to determine precise right-sided double-lumen tube position. A,
When the fiberoptic bronchoscope is passed down the left (tracheal) lumen, the endoscopist
should see a clear straight-ahead view of the tracheal carina and the right lumen
going off into the right main stem bronchus. B, When
the fiberoptic bronchoscope is passed down the right (bronchial) lumen, the endoscopist
should see the bronchial carina off in the distance; when the fiberoptic bronchoscope
is flexed cephalad and passed through the right upper lobe ventilation slot, the
right upper lobe bronchial orifice should be visualized. (From Benumof JL:
Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Insertion of the bronchial lumen of a DLT into the appropriate main stem bronchus may be aided by the use of a fiberoptic bronchoscope. It may be especially helpful if anatomic variation or a pathologic condition has caused carinal distortion. The DLT is first placed in the trachea in a conventional manner (laryngoscopy, manual tube insertion) until the tracheal cuff just passes the vocal cords, the tracheal cuff is inflated, and both lungs are ventilated through both lumens (the DLT should be used as though were a single-lumen tube). A pediatric-sized fiberoptic bronchoscope can then be inserted into the bronchial lumen through a self-sealing diaphragm in the elbow connector to the bronchial lumen (which permits continued positive-pressure ventilation through that lumen around the fiberoptic bronchoscope) and passed into the appropriate main stem bronchus. The tracheal cuff is then deflated and the bronchial lumen passed over the fiberoptic bronchoscope stylet into the appropriate main stem bronchus. The fiberoptic bronchoscope is then withdrawn from the bronchial lumen and passed down the tracheal lumen to determine the precise DLT position (see the preceding section).
Alternatively, once the DLT is in the trachea, the fiberoptic bronchoscope can be inserted into the tracheal lumen and passed just proximal to the tracheal carina. While the carina and the two main stem bronchial orifices are in view, the DLT can be advanced and the degree of lateral rotation adjusted so that the appropriate lumen enters the appropriate main stem bronchus. Final precise positioning (see the preceding section) can be done with the fiberoptic bronchoscope remaining in the tracheal lumen if a left-sided tube is used. If a right-sided tube is used, precise positioning must be confirmed with the bronchoscope passed through the bronchial lumen.
The clear plastic disposable right and left double-lumen endotracheal tubes are manufactured in four sizes: 35, 37, 39, and 41 French. In addition, 26 and 28 French tubes are available only as left-sided models. A diagnostic fiberoptic bronchoscope with a 5.6-mm outside diameter will not pass down the lumina of any size DLT. A fiberoptic bronchoscope with a 4.9-mm outside diameter passes easily through the lumens of the 41 French tube and moderately easily with lubrication through the 39 French tube; it causes a tight fit that needs a liberal amount of lubrication and a strong pushing force to pass through the lumen of the 37 French tube and does not pass through the lumen of the 35 French tube. A silicon-based fluid (such as that made by the American Cystoscope Company) is the best lubricant for a fiberoptic bronchoscope because it does not dry out or crust and does not interfere with the view even if it coats the tip of the bronchoscope. Fortunately, from the point of view of using a fiberoptic bronchoscope with a 4.9-mm outside diameter, a 37 French tube or larger can be used in almost all adult females and 39 French tube or larger in almost all adult males.
| Fiberoptic Bronchoscope Size (Outside Diameter) (mm) | Adult DLT Size (French) | Fit of Fiberoptic Bronchoscope inside DLT |
|---|---|---|
| 5.6 | All sizes | Does not fit |
|
|
41 | Easy passage |
|
|
39 | Moderately easy passage |
| 4.9 | 37 | Tight fit, need lubricant, * hard push |
|
|
35 | Does not fit |
| 3.6–4.2 | All sizes | Easy passage |
| DLT, double-lumen endotracheal tube. | ||
The chest radiograph can be used to determine DLT position. The chest radiograph may be more useful than conventional unilateral auscultation and clamping in some patients, but it is always less precise than fiberoptic bronchoscopy. To use the chest radiograph, the DLT must have radiopaque markers at the end of the right and left lumens. The key to discerning DLT position on the chest radiograph is seeing where the marker at the end of the tracheal lumen is in relation to the tracheal carina and whether the endobronchial lumen is located in the correct main stem bronchus. The end of the tracheal lumen marker must be above the tracheal carina; however, this does not guarantee correct position because this technique may not reveal subtle obstruction of an upper lobe. If the tracheal carina cannot be seen (as sometimes happens with a portable anteroposterior film), the chest radiograph method of determining DLT position is not usable. Furthermore, the chest radiograph method is time consuming (for film transport and film development), costly, and awkward to perform and may dislodge the tube (the cassettes are often difficult to place under the operating room table and require moving the patient).
Three other methods may help determine the position of a DLT. First, comparison of capnography (waveform and end-tidal CO2 pressure [PETCO2 ]) from each lumen may reveal a marked discrepancy. For example, with all other conditions being equal, one lung may be very poorly ventilated in relation to the other lung (high PETCO2 ), indicative of obstruction to that lung; one lung may be very overventilated in relation to the other lung (low PETCO2 ), perhaps indicative of ventilation of just a lobe of that lung; or the capnogram from one lung may have a much steeper slope to the alveolar plateau, indicative of expiratory obstruction.[264] [265] Second, continuous spirometric data (Datex Capnomac Ultima) from both lungs and from each lung separately, such as pressure-volume or flow-volume loops, may be displayed and compared with a control loop that is stored in memory.[266] Third, the surgeon may be able to palpate the position of the DLT from within the chest and may be able to redirect or assist in changing its position (by deflecting the DLT away from the wrong lung, etc.).[267]
The use of fiberoptic bronchoscopy to determine DLT position does not provide evidence or a guarantee that the lungs are functionally separated (i.e., against a fluid or air pressure gradient, or both). On occasion, such as during the performance of unilateral pulmonary lavage, the anesthesiologist must be absolutely certain that functional separation has been achieved. Complete separation of the lungs by the left endobronchial cuff can be demonstrated in a left-sided tube by clamping the connecting tube to the right lung proximal to the right suction port and attaching a small tube (i.e., intravenous extension tubing) to the open right suction port (by appropriate adaptors) ( Fig. 49-22 ). The free end of this tube is submerged in a beaker of water. When the left lung is statically inflated to any pressure considered necessary and the left endobronchial cuff is not sealed, air will enter the left lung and will also escape from around the unsealed left cuff, move up the right lumen to the small connecting tube, and bubble through the beaker of water ( Fig. 49-22B ). If the left endobronchial cuff is sealed, no bubbles should be observed passing through the beaker of water ( Fig. 49-22A ). After demonstration of functional lung separation, the right connecting tube is unclamped, the right suction port closed, and ventilation to both lungs resumed. To test for lung separation with the pressure gradient across the endobronchial balloon reversed, the left airway connecting tube is clamped proximal to the left suction port, the left suction port opened to the beaker of water through the small tube, the right lung statically inflated to any desired pressure, and the absence or presence of air bubbles in the beaker of water noted. It should be remembered that even though the left endobronchial cuff may be adequately sealed, it is possible that during these maneuvers, compression of the nonventilated lung by the ventilated lung may initially cause some small amount of bubbling in the beaker, which will cease with repetitive inflation of the ventilated lung (no bubbles should be seen after several inflations).[251] [260] The absence of airflow from the nonventilated lung suction port is a very simple, but sensitive indicator of functional separation of the two lungs.
In addition to the impediment to arterial oxygenation that is inherent in the use of DLTs for one-lung anesthesia, the
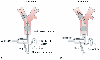 Figure 49-22
Schematic diagram showing the air bubble detection method
for checking adequacy of the seal of the left endobronchial cuff of a left-sided
double-lumen tube. A, When the left lung is selectively
ventilated or exposed to any desired distending pressure and the left cuff is adequately
sealed, no air will escape around the left cuff and out the open right suction port,
and thus no bubbles will be observed passing through the beaker of water. B,
When the left lung is ventilated or exposed to any desired distending pressure and
the left endobronchial cuff is not adequately sealed, air will escape around the
left cuff and out the open right suction port, and thus air bubbles will be observed
passing through the beaker of water. (From Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-22
Schematic diagram showing the air bubble detection method
for checking adequacy of the seal of the left endobronchial cuff of a left-sided
double-lumen tube. A, When the left lung is selectively
ventilated or exposed to any desired distending pressure and the left cuff is adequately
sealed, no air will escape around the left cuff and out the open right suction port,
and thus no bubbles will be observed passing through the beaker of water. B,
When the left lung is ventilated or exposed to any desired distending pressure and
the left endobronchial cuff is not adequately sealed, air will escape around the
left cuff and out the open right suction port, and thus air bubbles will be observed
passing through the beaker of water. (From Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
Lung separation by a DLT may be relatively contraindicated in several situations because insertion of the tube is either difficult or dangerous. These situations involve patients who have a full stomach (risk of aspiration), patients who have a lesion (airway stricture,[274] endoluminal tumor) that is present somewhere along the pathway of the DLT and thus could be traumatized, small patients for whom a 35 French tube is too large to fit comfortably through the larynx and for whom a 28 French tube is considered too small, patients whose upper airway anatomy precludes safe insertion of the tube (recessed jaw, prominent teeth, bull neck, anterior larynx), extremely critically ill patients who have a single-lumen tube already in place and who will not tolerate being taken off mechanical ventilation and PEEP for even a short time, and patients with some combination of these problems. Under these circumstances, it is still possible to separate the lungs safely and adequately by using a single-lumen tube and fiberoptic bronchoscopic placement of a bronchial blocker or by fiberoptic bronchoscopic placement of a single-lumen tube in a main stem bronchus.
Lung separation can be effectively achieved with the use of a
single-lumen endotracheal tube and a fiberoptically
| 1. Be particularly cautious in patients with bronchial wall abnormalities. |
| 2. Pick an appropriately sized tube. |
| 3. Be certain that the tube is not malpositioned. * Use fiberoptic bronchoscopy to confirm the position of the double-lumen tube (especially if N2 O is introduced into the inspired gases). |
| 4. Avoid overinflation of the endobronchial cuff. * |
| 5. Deflate the endobronchial cuff during turning. |
| 6. Inflate the endobronchial cuff slowly. |
| 7. Inflate the endobronchial cuff with inspired gases. |
| 8. Do not allow the tube to move during turning. * |
 Figure 49-23
Single-lumen tube of the Univent bronchial blocker (BB)
system.
Figure 49-23
Single-lumen tube of the Univent bronchial blocker (BB)
system.
The tube is inserted in the following manner. First, the single-lumen tube along with the bronchial blocker (in the fully retracted position) is inserted as a unit into the trachea ( Fig. 49-24A ). The cuff on the main endotracheal tube lumen is inflated, and the patient is ventilated and oxygenated (see Fig. 49-24A ). A fiberoptic bronchoscope is inserted through a self-sealing diaphragm in the elbow connector to a single-lumen tube while ventilation is maintained around the bronchoscope (but within the single-lumen tube) ( Fig. 49-24C ). The right and left main stem bronchi are identified by noting the relationship of the main stem bronchi to the posterior membrane and the anterior cartilaginous rings ( Fig. 49-24B ), and the tube of the bronchial blocker is located by moving the bronchial blocker in and out just beyond the end of its own lumen and the main lumens of the Univent tube ( Fig. 49-24 ). The bronchial blocker cuff is blue and easy to visualize. It will be seen that the bronchial blocker will usually (almost always) enter the right main stem bronchus if it is simply pushed in (and the main single-lumen tube is not turned). If the left main stem bronchus is to be blocked, the main single-lumen tube is turned 90 degrees to the left (counterclockwise) so that the concavity of the tube is facing toward the left side ( Fig. 49-24C ) (and vice versa for the right side, if necessary). The bronchial blocker can also be rotated a slight amount at its distal end (obtain 1 to 3 mm of laterality) by twirling the proximal end in the fingers. The bronchial blocker is then advanced into the main stem bronchus under direct vision ( Fig. 49-24D ). Attempting to advance the bronchial blocker blindly into the appropriate main stem bronchus (particularly the left one) will be unsuccessful 87% of the time, and repeated attempts may cause excoriation of the tracheal mucosa. [227] In fact, blindly pushing the somewhat stiff bronchial blocker may result in perforation of the tracheobronchial tree and consequent tension pneumothorax.[278] The balloon is inflated until the cephalad surface of the balloon is just below the tracheal carina ( Fig. 49-24E ) (so that the upper lobe of the blocked lung may also distend if CPAP is applied to the blocked lung [see later]), and the fiberoptic bronchoscope is then withdrawn ( Fig. 49-24F ).
The Univent bronchial blocker tube has six important attributes that require special mention ( Table 49-13 ). First and foremost, the degree of difficulty in inserting the Univent tube is equivalent to that of a standard single-lumen tube and in many instances will therefore be an easier and quicker means (than with a DLT) of separating the lungs to obtain simple one-lung ventilation.[91] [93] Thus, the Univent tube (or an independent bronchial blocker with conventional intubation—see later) is preferable when difficult intubation is anticipated. Second, the patient can be continuously ventilated while the bronchial blocker is being placed into a main stem bronchus, and the bronchial blocker can be placed into a main stem bronchus just as easily in the LDP as in the supine position. Third, and provided that the postanesthesia care unit and intensive care unit personnel are instructed in the design and function of the Univent tube (particularly the ventilatory consequence of inflating the bronchial blocker cuff just distal to the main lumen [i.e., the main lumen will be obstructed]), the Univent tube may be left in situ for postoperative mechanical ventilation and the risk of a potentially difficult tube change (e.g., from a DLT to a single-lumen tube) thereby avoided. Fourth and similarly, the Univent tube may be left in situ if a patient is turned from the supine to the prone position midway through a surgical procedure (a common occurrence with surgery on the thoracic spine). Fifth, the unique characteristics of a movable endobronchial blocker permit the Univent endotracheal tube to create selective partial collapse (e.g., of a lobe) or total collapse of the targeted lung. [282] The capability of selectively blocking lung segments is extremely important in cases of isolated
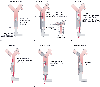 Figure 49-24
The sequential steps of the fiberoptic-aided method of
inserting and positioning the Univent bronchial blocker (BB) in the left main stem
bronchus are illustrated. One- or two-lung ventilation is achieved simply by inflating
or deflating, respectively, the bronchial blocker balloon. FOB, fiberoptic bronchoscope.
Figure 49-24
The sequential steps of the fiberoptic-aided method of
inserting and positioning the Univent bronchial blocker (BB) in the left main stem
bronchus are illustrated. One- or two-lung ventilation is achieved simply by inflating
or deflating, respectively, the bronchial blocker balloon. FOB, fiberoptic bronchoscope.
The Univent bronchial blocker tube system has several distinct
limitations, but
| 1. Easier to insert and properly position |
| 2. Can be properly positioned during continuous ventilation and in the lateral decubitus position |
| 3. No need to change the tube for postoperative mechanical ventilation |
| 4. No need to change the tube intraoperatively when turning from the supine to the prone position |
| 5. Selective blockade of some lobes of each lung |
| 6. Possible to apply nonventilated operative lung CPAP |
| CPAP, continuous positive airway pressure. |
| Limitation | Solution |
|---|---|
| Slow deflation time | (a) Deflate the bronchial blocker cuff and compress and evacuate the lung through the main single lumen; (b) apply suction to the bronchial blocker lumen |
| Slow reinflation time | (a) Deflate the bronchial blocker cuff and administer a positive-pressure breath through the main single lumen; (b) carefully administer one short high-pressure (20–30 psi) jet ventilation breath |
| Blockage of the bronchial blocker lumen by blood, pus | Suction, stylet, and then suction |
| High-pressure cuff | Use a just-seal volume of air |
| Intraoperative leak in the bronchial blocker cuff | Make sure that the bronchial blocker cuff is subcarinal, increase the inflation volume, rearrange the surgical field |
Second, reinflation of the lung is extremely tedious with the balloon inflated. The operative lung is most safely made to expand rapidly by deflating the bronchial blocker cuff (the operative lung will expand with one positive-pressure breath from the main single lumen) or by administering one very short (e.g., <0.5 second) burst of wall oxygen-powered 20- to 30-psi jet ventilation (reduced from 50 psi). However, connection of the bronchial blocker lumen to a jet ventilator is potentially dangerous (i.e., it can cause barotrauma) because the lung can expand extremely rapidly, and it is of paramount importance that the anesthesiologist directly observe the lung and that the ventilation be very short or the pressure be limited to 20 to 30 psi by an additional in-line regulator.
Third, and also because the bronchial blocker lumen is small, the lumen is relatively easily blocked by blood or pus, or both. High suction will occasionally clear the lumen of these materials, and total blockage by inspissated secretions can be broken up by a wire stylet. Fourth, the Univent bronchial blocker behaves as a high-pressure cuff when the intracuff volume is larger than 2 mL (the resting volume of the cuff) and may be expected to have an intracuff pressure between 150 and 250 mm Hg and a transmural pressure (intracuff pressure within the airway minus intracuff pressure outside the airway [free in the room]) between 50 and 60 mm Hg when intracuff volumes of 4 to 6 mL are used to seal 12- to 18-mm airways against the usual proximal airway pressure.[284] [285] Thus, the order of usual bronchial cuff pressures is left-sided PVC DLT < right-sided PVC DLT < Univent bronchial blocker cuff < red rubber double-lumen tube.[286] These findings underscore the need to inflate the bronchial blocker cuff with a just-seal volume of air. Fifth, the Univent bronchial blocker has on occasion been reported to have a minor leak during surgery (25% in one series),[277] but this is not understandable in view of experiments showing that the Univent bronchial blocker cuff seals within normal-sized main stem bronchi against proximal airway pressures as great as 100 cm H2 O with inflation volumes that are within the manufacturer's recommendation.[285] Consequently, if an intraoperative leak occurs when less than a 6- to 7-mL intracuff volume has been used and the bronchial blocker cuff is completely subcarinal (as determined by fiberoptic bronchoscopy) and intact, the intracuff volume should be increased. If an intraoperative leak develops even though an adequate cuff inflation volume has been used (the bronchial blocker can be seen [fiberoptically] to fill the main stem bronchus in question) and the bronchial blocker cuff is completely subcarinal (as determined fiberoptically) and intact, the relationship between the main stem bronchus and the bronchial blocker cuff may no longer be a simple matter of a sphere or ellipsoid being inflated within a cylinder. Under these circumstances, the surgeon may need to rearrange the surgical field so that the main stem bronchus and bronchial blocker cuff are less distorted. Finally, the addition of the lumen for the bronchial blocker results in an endotracheal tube that has a large outside anteroposterior diameter relative to its inside diameter.
Two methods can be used to obtain a just-seal volume of air in the bronchial blocker cuff. The first method is the same as that already described for obtaining a just-seal volume of air in the endobronchial cuff of a DLT. It consists of pressurizing the main single lumen until air ceases to escape from the bronchial blocker lumen (detected by connecting the bronchial blocker lumen to a catheter that is submerged beneath the surface of a beaker of water; when air bubbles cease to come out, the bronchial blocker cuff has sealed) (see Fig. 49-22 ).
The second method appears promising and uses capnography. End-tidal CO2 analyzers draw gas samples from the anesthesia breathing circuit through tubing that terminates, at the patient end of the tubing, in a standard Luer-Lock male connector that inserts into a female port in the breathing circuit. The male connector also inserts into/attaches to the female port at the proximal end of
In several clinical situations, use of the Univent bronchial blocker tube is relatively indicated (versus a DLT). However, independent bronchial blockers (see later) can perform to the same degree or in some areas better (e.g., critically ill, already intubated patients) than the Univent. Nonetheless, the following clinical conditions warrant consideration of a Univent tube. First, whenever it is anticipated that postoperative ventilation will be necessary (e.g., poor pulmonary function preoperatively, anticipated lung damage or massive fluid or blood infusion intraoperatively, anticipated very long procedure), use of the Univent bronchial blocker tube for lung separation may avoid a risky postoperative change from a DLT to a single-lumen tube. Second and similarly, use of the Univent bronchial blocker tube will avoid a potentially
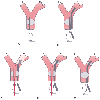 Figure 49-25
Lung separation with a single-lumen tube, fiberoptic
bronchoscope, and right lung bronchial blocker. The sequence of events is as follows.
A, A single-lumen tube is inserted and the patient
is ventilated. B, A bronchial blocker is passed alongside
the indwelling endotracheal tube. C, A fiberoptic
bronchoscope is passed through a self-sealing diaphragm in the elbow connecter to
the endotracheal tube and is used to place the bronchial blocker into the right main
stem bronchus under direct vision. D, The balloon
on the bronchial blocker is also inflated under direct vision and is positioned just
below the tracheal carina. E, The fiberoptic bronchoscope
is then removed. During the lower-panel sequence
(insertion and use of the fiberoptic bronchoscope ([C
to E]), the self-sealing diaphragm allows the patient
to continue to be ventilated with positive-pressure ventilation (around the fiberoptic
bronchoscope, but within the lumens of the endotracheal tube). LL, left lung; RL,
right lung. (From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia,
WB Saunders, 1987.)
Figure 49-25
Lung separation with a single-lumen tube, fiberoptic
bronchoscope, and right lung bronchial blocker. The sequence of events is as follows.
A, A single-lumen tube is inserted and the patient
is ventilated. B, A bronchial blocker is passed alongside
the indwelling endotracheal tube. C, A fiberoptic
bronchoscope is passed through a self-sealing diaphragm in the elbow connecter to
the endotracheal tube and is used to place the bronchial blocker into the right main
stem bronchus under direct vision. D, The balloon
on the bronchial blocker is also inflated under direct vision and is positioned just
below the tracheal carina. E, The fiberoptic bronchoscope
is then removed. During the lower-panel sequence
(insertion and use of the fiberoptic bronchoscope ([C
to E]), the self-sealing diaphragm allows the patient
to continue to be ventilated with positive-pressure ventilation (around the fiberoptic
bronchoscope, but within the lumens of the endotracheal tube). LL, left lung; RL,
right lung. (From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia,
WB Saunders, 1987.)
The Fogarty vascular embolectomy catheter is a device designed specifically for vascular surgery; however, it has long been used as an independent (of a single-lumen tube) bronchial blocker for lung separation.[243] The common sizes of Fogarty catheter used for bronchial blockade include 6.0, 8/14, and 8/22 catheters. The number 8 refers to the catheter size deflated (in French sizing), and the numbers 14 and 22 refer to the size of the inflated balloon diameter (in millimeters). The Fogarty catheter includes a stylet so that it is possible to place a curvature at the distal tip to facilitate entry into the larynx and either main stem bronchus (by twirling the proximal end). If no endotracheal tube is in place, the operator exposes the larynx and places a single-lumen tube with a high-volume cuff in the trachea. The Fogarty catheter is then placed either inside[287] or alongside the single-lumen tube ( Fig. 49-25 ). In either case (bronchial blocker inside or outside the
 Figure 49-26
The Arndt bronchial blocker kit includes a wire-guided
endobronchial blocker and a multiport airway adapter. After placement of an endotracheal
tube, the Arndt bronchial blocker is placed through the blocker port of the airway
adapter; the fiberoptic bronchoscope is then placed through the wire loop, into the
trachea, and positioned within the bronchus of choice. The fiberoptic bronchoscope
has to be advanced far enough so that the Arndt blocker will enter the bronchus while
it is being advanced. Once the deflated cuff is below the entrance of the bronchus,
the fiberoptic bronchoscope is withdrawn, and the cuff is fully inflated with air
(using the just-seal approach). After the patient is turned to the lateral decubitus
position, bronchoscopic confirmation is necessary to ensure that the cuff of the
Arndt blocker is still properly positioned.
Figure 49-26
The Arndt bronchial blocker kit includes a wire-guided
endobronchial blocker and a multiport airway adapter. After placement of an endotracheal
tube, the Arndt bronchial blocker is placed through the blocker port of the airway
adapter; the fiberoptic bronchoscope is then placed through the wire loop, into the
trachea, and positioned within the bronchus of choice. The fiberoptic bronchoscope
has to be advanced far enough so that the Arndt blocker will enter the bronchus while
it is being advanced. Once the deflated cuff is below the entrance of the bronchus,
the fiberoptic bronchoscope is withdrawn, and the cuff is fully inflated with air
(using the just-seal approach). After the patient is turned to the lateral decubitus
position, bronchoscopic confirmation is necessary to ensure that the cuff of the
Arndt blocker is still properly positioned.
The Arndt wire-guided endobronchial blocker ( Fig.
49-26
) is a relatively new product for lung separation that combines ease
of positioning (wire guided), less potential for mucosal injury (a low-pressure high-volume
cuff), a distal lumen (useful for suction, lung deflation, and CPAP), and
| Size (French) | Length (cm) | Cuff Shape | Cuff Inflation Volume (mL) | "Murphy Eye" Side Holes | Smallest SLT ID for Coaxial Use (mm) |
|---|---|---|---|---|---|
| 9 | 78 and 65 | Elliptical and spherical | 6–12 | Yes | 7.5 |
|
|
|
|
4–8 |
|
|
| 7 | 65 | Spherical | 2–6 | No | 6.0 |
| 5 | 65 and 50 | Spherical | 0.5–2.0 | No | 4.5 |
| ID, inner diameter; SLT, single lumen tube. | |||||
The technique for lung separation using the Arndt wire-guided endobronchial catheter is as follows. Before insertion,
The advantage of the Arndt bronchial blocker over a Fogarty is that (like a DLT) lung separation includes the ability to suction or ventilate (or both) the lung distal to the blocker, with minimal increased placement time. The benefit of the Arndt bronchial blocker over both the Univent and a DLT is that it can be used in patients who are already intubated,[288] those with a difficult airway,[289] and those requiring lung separation for trauma.[290] A disadvantage of any bronchial blocker over a DLT is that if a main stem bronchial blocker backs out into the trachea, the seal between the two lungs will be lost, and two catastrophic complications may occur. First, if the bronchial blocker was being used to seal off fluid (blood or pus) in one lung, both lungs may become contaminated with the fluid. Second, the trachea will be at least partially obstructed by the blocker, and ventilation will be greatly impaired. Therefore, bronchial blockage requires that the anesthetist continuously and intensively monitor the compliance and breath sounds of the ventilated lung.
In adults with hemoptysis, endobronchial intubation with a single-lumen tube is often the easiest, quickest way of effectively separating the two lungs, especially if the left lung is bleeding, in which case one can simply take an uncut single-lumen endotracheal tube and advance it inward until moderate resistance is felt. In the vast majority of patients, the single-lumen tube will be located in the right main stem bronchus, thereby blocking off the bleeding left lung and allowing selective ventilation of only the right lung. In these circumstances it is highly possible that the right upper lobe bronchus will be blocked off as well, with resultant ventilation of only the right middle and lower lobes. Ventilation of only a soiled right lung or ventilation of only the right middle and lower lobes (even if they are unsoiled) incurs the risk of serious hypoxemia as a result of the very large transpulmonary shunt that is necessarily created by single-lung endobronchial intubation.
If the right lung is bleeding, there are two ways of selectively intubating the left main stem bronchus. First, intubation may be done blindly with an approximately 92% success rate by turning the patient's head to the right and passing the single-lumen tube with the concavity of the tube facing posteriorly (rotated 180 degrees from its normal position and relationship to the trachea).[291] The single-lumen tube enters the right or left main stem bronchus when the concavity of the tube faces anteriorly or posteriorly, respectively, because the bevel is left or right facing, respectively (i.e., a left-facing bevel enters the right main stem bronchus and a right-facing bevel enters the left main stem bronchus).[292] Second, a fiberoptic bronchoscope can be passed through a self-sealing diaphragm in the elbow connector of the single-lumen tube and directed into the left main stem bronchus. Persistent large, soft catheter suctioning of the carinal area through the single-lumen tube before use and suctioning through the fiberoptic bronchoscope (through the single-lumen tube) may be required to visualize the tracheal carina. The single-lumen tube can then be passed over the fiberoptic bronchoscope into the left main stem bronchus, thereby isolating the bleeding right lung and allowing selective ventilation of the left lung. Passing the fiberoptic bronchoscope through a self-sealing diaphragm allows continuance of positive-pressure ventilation and PEEP around the bronchoscope. However, it should be realized that visualization of the carina may not be possible when the bleeding is copious and that the only hope for the patient may lie in rapid thoracotomy and control of bleeding from within the chest. In addition, under these adverse conditions, conventional passage of a DLT may more rapidly and effectively separate the two lungs than visualization of the anatomy with a fiberoptic bronchoscope.
In summary, the use of DLTs is the method of choice for separating the lungs in most adult patients. If any question arises, the precise location of a DLT can be determined by fiberoptic bronchoscopy at any time. In a number of situations, insertion of a DLT may be difficult or dangerous, or both, and under these circumstances consideration should be given to separating the lungs with a single-lumen tube alone or in combination with a bronchial blocker (e.g., the Univent tube). However, when using a single-lumen tube in a main stem bronchus or when using a bronchial blocker, the ability to suction the operative site and control oxygen uptake (the blocked
As discussed previously, matching of ventilation and perfusion is impaired during two-lung ventilation in an anesthetized, paralyzed, open-chest patient in the LDP. The reason for the mismatching is relatively good ventilation but poor perfusion of the nondependent lung and poor ventilation and good perfusion of the dependent lung (see Fig. 49-13 and Fig. 49-26A ). The blood flow distribution has been noted to be mainly and simply determined by gravitational effects. The relatively good ventilation of the nondependent lung has been seen to be caused, in part, by the open chest and paralysis. The relatively poor ventilation of the dependent lung has been noted to be caused, in part, by the loss of dependent lung volume with general anesthesia and by circumferential compression of the dependent lung by the mediastinum, abdominal contents, and suboptimal positioning effect. Compression of the dependent lung may cause the development of a shunt compartment in this lung (see Fig. 49-13 ; Fig. 49-27A ). Consequently, two-lung ventilation under
 Figure 49-27
Schematic representation of two-lung ventilation versus
one-lung ventilation. Typical values for fractional blood flow to the nondependent
and dependent lungs are shown, as well as arterial oxygen tension (PaO2
)
and shunt (QS/QT)
for the two conditions. QS/QT
during two-lung ventilation is assumed to be distributed equally between the two
lungs (5% to each lung). The essential difference between two-lung and one-lung
ventilation is that during one-lung ventilation, the nonventilated lung has some
blood flow and therefore has an obligatory shunt, which is not present during two-lung
ventilation. The 35% of total flow perfusing the nondependent lung, which was not
shunt flow, was assumed to be able to reduce its blood flow by 50% by hypoxic pulmonary
vasoconstriction. The increase in QS/QT
from two-lung to one-lung ventilation is assumed to be solely due to the increase
in shunt through the nonventilated, nondependent lung during one-lung ventilation.
(From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders,
1987.)
Figure 49-27
Schematic representation of two-lung ventilation versus
one-lung ventilation. Typical values for fractional blood flow to the nondependent
and dependent lungs are shown, as well as arterial oxygen tension (PaO2
)
and shunt (QS/QT)
for the two conditions. QS/QT
during two-lung ventilation is assumed to be distributed equally between the two
lungs (5% to each lung). The essential difference between two-lung and one-lung
ventilation is that during one-lung ventilation, the nonventilated lung has some
blood flow and therefore has an obligatory shunt, which is not present during two-lung
ventilation. The 35% of total flow perfusing the nondependent lung, which was not
shunt flow, was assumed to be able to reduce its blood flow by 50% by hypoxic pulmonary
vasoconstriction. The increase in QS/QT
from two-lung to one-lung ventilation is assumed to be solely due to the increase
in shunt through the nonventilated, nondependent lung during one-lung ventilation.
(From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders,
1987.)
If the nondependent lung is nonventilated, as during one-lung ventilation, any blood flow to the nonventilated lung becomes shunt flow, in addition to whatever shunt flow might exist in the dependent lung ( Fig. 49-27B ). Thus, one-lung ventilation creates an obligatory right-to-left transpulmonary shunt through the nonventilated nondependent lung that is not present during two-lung ventilation. Consequently, it is not surprising to find that given the same inspired oxygen concentration (FIO2 ) and hemodynamic and metabolic status, one-lung ventilation results in a much larger PAO2 -PaO2 gradient and lower PaO2 than two-lung ventilation does. This contention is best supported by one study that compared arterial oxygenation during two-lung and one-lung ventilation, wherein the patients served as their own controls.[293]
One-lung ventilation has much less of a steady-state effect on PaCO2 than on PaO2 . Blood passing through under-ventilated alveoli retains more than a normal amount of carbon dioxide and does not take up a normal amount of oxygen; blood traversing overventilated alveoli gives off more than a normal amount of carbon dioxide but cannot take up a proportionately increased amount of oxygen because of the flatness of the top end of the oxyhemoglobin dissociation curve. Thus, during one-lung ventilation (one-lung minute ventilation equals two-lung minute ventilation), the ventilated lung can eliminate enough carbon dioxide to compensate for the nonventilated lung, and PACO2 to PaCO2 gradients are small; however, the ventilated lung cannot take up enough oxygen to compensate for the nonventilated lung, and PAO2 to PaO2 gradients are usually large. With constant minute ventilation (two-lung ventilation versus one-lung ventilation), retention of carbon dioxide by blood traversing the nonventilated
The initiation of one-lung ventilation has much more of an acute
effect (first 5 minutes) on PETCO2
. When
one-lung ventilation is begun (keeping total tidal volume and the respiratory rate
constant), the ventilated lung is immediately hyperventilated in relation to its
perfusion (i.e., has an increased V̇/![]() ratio), and PETCO2
from this lung decreases in the first minute (e.g., by 5 mm Hg).[294]
ratio), and PETCO2
from this lung decreases in the first minute (e.g., by 5 mm Hg).[294]
Over the next 5 minutes, HPV in the nonventilated lung shifts
blood flow over to the ventilated lung, increases perfusion of the ventilated lung,
decreases the V̇/![]() ratio of the ventilated lung, and increases PETCO2
back to the baseline two-lung ventilation value.[294]
Thereafter, and as discussed previously, PETCO2
will slowly increase (along with PaCO2
)
because the same total minute ventilation to one lung is not as effective as when
it is delivered to both lungs (i.e., alveolar dead space is increased within the
one ventilated lung).[294]
ratio of the ventilated lung, and increases PETCO2
back to the baseline two-lung ventilation value.[294]
Thereafter, and as discussed previously, PETCO2
will slowly increase (along with PaCO2
)
because the same total minute ventilation to one lung is not as effective as when
it is delivered to both lungs (i.e., alveolar dead space is increased within the
one ventilated lung).[294]
Fortunately, both passive mechanical and active vasoconstrictor mechanisms are usually operant during one-lung ventilation to minimize blood flow to the nondependent, nonventilated lung and thereby prevent PaO2 from decreasing as much as might be expected on the basis of the distribution of blood flow during two-lung ventilation. The passive mechanical mechanisms that decrease blood flow to the nondependent lung are gravity, surgical interference with blood flow, and perhaps the extent of preexisting disease in the nondependent lung ( Fig. 49-28 ). Gravity causes a vertical gradient in the distribution of pulmonary blood flow in the LDP for the same reason that it does in the upright position (see Fig. 49-9 ). Consequently, blood flow to the nondependent lung is less than that to the dependent lung. The gravity component of blood flow reduction to the nondependent lung should be constant with respect to both time and magnitude.
 Figure 49-28
Schematic diagram of the determinants of blood flow distribution
during one-lung ventilation. The major determinants of blood flow to the nondependent
lung are gravity, surgical interference with blood flow, amount of nondependent lung
disease, and magnitude of nondependent lung hypoxic pulmonary vasoconstriction.
The determinants of dependent lung blood flow are gravity, amount of dependent lung
disease, and dependent lung hypoxic pulmonary vasoconstriction. RV, right ventricle.
(From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders,
1987.)
Figure 49-28
Schematic diagram of the determinants of blood flow distribution
during one-lung ventilation. The major determinants of blood flow to the nondependent
lung are gravity, surgical interference with blood flow, amount of nondependent lung
disease, and magnitude of nondependent lung hypoxic pulmonary vasoconstriction.
The determinants of dependent lung blood flow are gravity, amount of dependent lung
disease, and dependent lung hypoxic pulmonary vasoconstriction. RV, right ventricle.
(From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders,
1987.)
Surgical compression (directly compressing lung vessels) and retraction (causing kinking and tortuosity of lung vessels) of the nondependent lung may further passively reduce nondependent lung blood flow. In addition, ligation of pulmonary vessels during pulmonary resection greatly decreases nondependent lung blood flow. The surgical interference component of blood flow reduction to the nondependent lung should be variable with respect to both time and magnitude.
The amount of disease in the nondependent lung is also a significant determinant of the amount of blood flow to the nondependent lung. If the nondependent lung is severely diseased, there may be a fixed reduction in blood flow to this lung preoperatively, and collapse of such a diseased lung may not cause much of an increase in shunt. The notion that diseased pulmonary vasculature might be incapable of HPV is supported by the observations that administration of sodium nitroprusside and nitroglycerin (which should abolish any preexisting HPV) to COPD patients (who have a fixed reduction in the cross-sectional area of their pulmonary vascular bed) does not cause an increase in shunt[295] whereas these drugs do increase shunt in patients with acute regional lung disease who have an otherwise normal pulmonary vascular bed.[296] If the nondependent lung is normal and has a normal amount of blood flow preoperatively, collapse of such a normal lung may be associated with higher blood flow and shunt to the nonventilated nondependent lung. A higher one-lung ventilation shunt through the nondependent lung may be more likely to occur in patients who require thoracotomy for nonpulmonary disease.[297] Two studies have systematically validated the inverse correlation between the amount of nondependent lung disease and shunt during one-lung ventilation.[298] [299]
The most significant reduction in blood flow to the nondependent lung is caused by an active vasoconstrictor mechanism. The normal response of the pulmonary vasculature to atelectasis is an increase in PVR (in just the atelectatic lung); this increase, thought to be due almost entirely to HPV,[153] [154] [155] diverts blood flow from the atelectatic lung toward the remaining normoxic or hyperoxic ventilated lung. The diversion of blood flow minimizes the
Figure 49-29 outlines the major determinants of the amount of atelectatic lung HPV that might occur during anesthesia. In the following discussion, the HPV issues or considerations are numbered as they are in Figure 49-29 .
 Figure 49-29
Listing of many of the components of the anesthetic experience
that might determine the amount of regional hypoxic pulmonary vasoconstriction.
The clockwise numbering of considerations corresponds to the order in which these
considerations are discussed in the text. FIO2
,
inspired oxygen fraction; OaCO2
, alveolar
carbon dioxide tension; PEEP, positive end-expiratory pressure; Pv̄O2
,
mixed venous oxygen tension; PVP, pulmonary vascular pressure; V̇/
Figure 49-29
Listing of many of the components of the anesthetic experience
that might determine the amount of regional hypoxic pulmonary vasoconstriction.
The clockwise numbering of considerations corresponds to the order in which these
considerations are discussed in the text. FIO2
,
inspired oxygen fraction; OaCO2
, alveolar
carbon dioxide tension; PEEP, positive end-expiratory pressure; Pv̄O2
,
mixed venous oxygen tension; PVP, pulmonary vascular pressure; V̇/![]() , ventilation-perfusion
ratio. (From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia,
WB Saunders, 1987.)
, ventilation-perfusion
ratio. (From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia,
WB Saunders, 1987.)
Finally, some evidence has indicated that certain types of infections that may cause atelectasis, particularly granulomatous and pneumococcal infections, may inhibit HPV.[363] [364]
The dependent lung usually has an increased amount of blood flow
because of both passive gravitational effects and active nondependent lung vasoconstrictor
effects. However, the dependent lung may also have a hypoxic compartment (area of
low V̇/![]() and atelectasis) that was present preoperatively or that developed
intraoperatively. This hypoxic compartment may develop intraoperatively for several
reasons. First, in the LDP the ventilated dependent lung usually has a reduced lung
volume resulting from the combined factors of induction of general anesthesia and
circumferential (and perhaps severe) compression by the mediastinum from above, by
the abdominal contents pressing against the diaphragm from the caudad side, and by
suboptimal positioning effects (rolls, packs, chest supports) pushing in from the
dependent side and axilla (see Fig.
49-13
).[207]
[231]
[234]
[365]
Second,
absorption atelectasis can also occur in regions of the dependent lung that have
low V̇/
and atelectasis) that was present preoperatively or that developed
intraoperatively. This hypoxic compartment may develop intraoperatively for several
reasons. First, in the LDP the ventilated dependent lung usually has a reduced lung
volume resulting from the combined factors of induction of general anesthesia and
circumferential (and perhaps severe) compression by the mediastinum from above, by
the abdominal contents pressing against the diaphragm from the caudad side, and by
suboptimal positioning effects (rolls, packs, chest supports) pushing in from the
dependent side and axilla (see Fig.
49-13
).[207]
[231]
[234]
[365]
Second,
absorption atelectasis can also occur in regions of the dependent lung that have
low V̇/![]() ratios when they are exposed to a high inspired oxygen concentration.
[366]
[367]
Third,
difficulty in removal of secretions may cause the development of poorly ventilated
and atelectatic areas in the dependent lung. Finally, maintaining the LDP for prolonged
periods may cause fluid to transude into the dependent lung (which may be vertically
below the left atrium) and cause a further decrease in lung volume and an increase
in airway closure in the dependent lung.[368]
ratios when they are exposed to a high inspired oxygen concentration.
[366]
[367]
Third,
difficulty in removal of secretions may cause the development of poorly ventilated
and atelectatic areas in the dependent lung. Finally, maintaining the LDP for prolonged
periods may cause fluid to transude into the dependent lung (which may be vertically
below the left atrium) and cause a further decrease in lung volume and an increase
in airway closure in the dependent lung.[368]
The development of a low V̇/![]() ratio or atelectatic areas,
or both, in the dependent lung increases vascular resistance
ratio or atelectatic areas,
or both, in the dependent lung increases vascular resistance
Still other factors may contribute to hypoxemia during one-lung ventilation. Hypoxemia from mechanical failure of the oxygen supply system or the anesthesia machine is a recognized hazard of any kind of anesthesia. Gross hypoventilation of the dependent lung can be a major cause of hypoxemia. Malfunction of the airway lumen of the dependent lung (blockage by secretions) and malposition of the DLT are, in our experience, frequent causes of increased PAO2 -PaO2 and hypoxemia. Resorption of residual oxygen from the nonventilated lung is time dependent and accounts for a gradual increase in shunt and decrease in PaO2 after one-lung ventilation is initiated.[369] With all other anesthetic and surgical factors constant, anything that decreases Pv̄O2 (decreased cardiac output, increased oxygen consumption [excessive stimulation of the sympathetic nervous system, hyperthermia, shivering]) causes increased PAO2 -PaO2 . [372] [373] Finally, transfusion of blood may cause pulmonary dysfunction, and this dysfunction has been attributed to the action of isoantibodies against leukocytes, which causes cellular aggregation, microvascular occlusion, and capillary leakage. Indeed, such a reaction has been described during prolonged one-lung ventilation.[374] Interestingly, the noncollapsed lung was preferentially injured, and the collapsed lung showed only minimal radiologic signs of edema after reexpansion.[374]
The proper initial conventional management of one-lung ventilation is logically based on the preceding determinants of blood flow distribution during one-lung ventilation. In view of the fact that one-lung ventilation incurs a definite risk of systemic hypoxemia, it is extremely important that dependent lung ventilation, as it affects these determinants, be optimally managed. This section considers the usual management of one-lung ventilation in terms of the most appropriate FIO2 , tidal volume, and respiratory rate ( Table 49-16 ).
| Maintain two-lung ventilation as long as possible. |
| Use FIO2 of 1.0 |
| Begin one-lung ventilation with a tidal volume of 8–10 mL/kg |
| Adjust the respiratory rate so that PaCO2 = 40 mm Hg |
| Use continuous monitoring of oxygenation and ventilation |
Although the theoretical possibilities of absorption atelectasis and oxygen toxicity exist, the benefits of ventilating the dependent lung with 100% oxygen far exceed the risks. A high FIO2 in the single ventilated lung may critically increase PaO2 from arrhythmogenic and life-threatening levels to safer levels.
In addition, high FIO2 in the dependent lung causes vasodilation, thereby increasing the dependent lung's capability of accepting blood flow redistribution as a result of nondependent lung HPV. Direct chemical 100% oxygen toxicity does not occur during the operative period, [375] and absorption atelectasis in the dependent lung[375] is unlikely to occur in view of the remaining one-lung ventilation management characteristics (moderately large tidal volumes with intermittent positive-pressure, low-level PEEP). If the anesthesiologist is concerned about and wishes to limit FIO2 in patients treated with either bleomycin or mitomycin, it is possible to carefully increase or decrease FIO2 to both lungs independently in an attempt to deliver the lowest FIO2 to both lungs (even when CPAP is administered to the nonventilated lung).[376]
The dependent lung should be ventilated with a tidal volume of approximately 8 to 10 mL/kg. A much smaller tidal volume might promote atelectasis of the dependent lung; a much greater tidal volume might excessively increase airway pressure and vascular resistance in the dependent lung[369] and thereby increase nondependent lung blood flow (decrease nondependent lung HPV). [360] [377] [378] If a tidal volume of 8 to 10 mL/kg causes excessive airway pressure, it should be lowered (after mechanical causes [i.e., tube malfunction] have been ruled out) and the respiratory rate increased (see later).
A dependent lung tidal volume of 8 to 10 mL/kg is in the lower end of the range of tidal volumes (8 to 15 mL/kg) that have been found in one study to not affect arterial oxygenation greatly during one-lung ventilation.[302] In that study, changes in PaO2 with alterations in tidal volume (during stable one-lung ventilation conditions) in individual patients were variable and unpredictable in both degree and direction (although the mean value for the group did not change). Thus, it appears that changing the tidal volume from 15 to 8 mL/kg during one-lung ventilation has an unpredictable, but not generally great impact on arterial oxygenation.[302]
No or just a very low level of dependent lung PEEP (<5 cm H2 O) should be used initially because of concern of unnecessarily increasing dependent lung PVR. Although increased PVR is unlikely to occur when dependent lung PEEP is less than 5 cm H2 O,[379] the presence of intrinsic PEEP during one-lung ventilation may make the total PEEP excessive (see the section "Selective Dependent Lung PEEP").[380]
The respiratory rate should be set so that PaCO2 remains at 40 mm Hg. Because a dependent lung tidal volume of 10 mL/kg represents a 20% decrease from the usual two-lung tidal volume of 12 mL/kg, the respiratory rate usually has to be increased by 20% to 30% to maintain carbon
In summary, at the commencement of one-lung ventilation, 100% oxygen, a tidal volume of 8 to 10 mL/kg, and a 20% increase in respiratory rate are used as the initial ventilation settings (see Table 49-16 ). Ventilation and arterial oxygenation are monitored by arterial blood gases, the end-tidal carbon dioxide concentration, and pulse oximetry. If there is a problem with either ventilation or arterial oxygenation, one or more of the differential lung management techniques described next are used.
 Figure 49-30
Four-part schematic diagram showing the effects of various
differential lung management approaches. A, The one-lung
ventilation situation. The down (dependent) lung
is ventilated (Vent) but is compressed by the weight of the mediastinum (M) from
above, by the pressure of the abdominal contents against the diaphragm (D), and by
the positioning effects of rolls, packs, and shoulder supports (P). The up
(nondependent) lung is nonventilated (Nonvent), and blood flow through this lung
is shunt flow. B, The dependent lung has been selectively
treated with positive end-expiratory pressure (PEEP), which improves V̇/
Figure 49-30
Four-part schematic diagram showing the effects of various
differential lung management approaches. A, The one-lung
ventilation situation. The down (dependent) lung
is ventilated (Vent) but is compressed by the weight of the mediastinum (M) from
above, by the pressure of the abdominal contents against the diaphragm (D), and by
the positioning effects of rolls, packs, and shoulder supports (P). The up
(nondependent) lung is nonventilated (Nonvent), and blood flow through this lung
is shunt flow. B, The dependent lung has been selectively
treated with positive end-expiratory pressure (PEEP), which improves V̇/![]() relationships in the dependent lung but also increases dependent lung vascular resistance;
this situation diverts blood to and thereby increases shunt flow through the nonventilated
lung. C, Selective application of continuous positive
airway pressure (CPAP) to the nondependent lung permits oxygen uptake from this lung;
even if CPAP causes an increase in vascular resistance and diverts blood flow to
the dependent lung, the diverted blood flow can still participate in gas exchange
in the ventilated dependent lung. Consequently, selective nondependent lung CPAP
can greatly increase PaO2
. D,
With differential lung CPAP (nondependent lung)/PEEP (dependent lung), it does not
matter where the blood flow goes because both lungs can participate in O2
uptake. With this latter one-lung ventilation pattern, PaO2
can be restored to levels near those achieved by two-lung ventilation.
relationships in the dependent lung but also increases dependent lung vascular resistance;
this situation diverts blood to and thereby increases shunt flow through the nonventilated
lung. C, Selective application of continuous positive
airway pressure (CPAP) to the nondependent lung permits oxygen uptake from this lung;
even if CPAP causes an increase in vascular resistance and diverts blood flow to
the dependent lung, the diverted blood flow can still participate in gas exchange
in the ventilated dependent lung. Consequently, selective nondependent lung CPAP
can greatly increase PaO2
. D,
With differential lung CPAP (nondependent lung)/PEEP (dependent lung), it does not
matter where the blood flow goes because both lungs can participate in O2
uptake. With this latter one-lung ventilation pattern, PaO2
can be restored to levels near those achieved by two-lung ventilation.
Intermittent inflation of the collapsed lung with oxygen during one-lung ventilation may be expected to increase PaO2 for a variable period. In a group of thoracic surgery patients undergoing one-lung ventilation with an inspired nitrous oxide fraction (FIN2 O) of 0.5 and an FIO2 of 0.5, the collapsed lung was manually inflated with a breath every 5 minutes with 2 L of oxygen and the lung was then allowed to collapse again; PaO2 increased by more than 28 mm Hg after each inflation.[384] The beneficial effect of each inflation persisted to a large extent to the next breath, even if at a gradually decreasing level. Although PaO2 decreased between inflations, it never reached the level observed in controls (no lung inflation) during 19 minutes of one-lung ventilation.
Because the ventilated dependent lung often has a decreased lung volume during one-lung ventilation (see Fig. 49-13 ,
Low levels of positive pressure can be selectively and statically applied to only the nonventilated, nondependent lung. Because under these conditions the nonventilated lung is only slightly, but constantly distended by oxygen, an appropriate term for this ventilatory arrangement is nonventilated lung CPAP. The application of CPAP (without tidal ventilation) to only the nonventilated lung significantly increases oxygenation.[388] [391] In addition, institution of 10 cm H2 O CPAP in the nondependent lung of patients has no significant hemodynamic effect.[385] [389] Low levels of CPAP simply maintain the patency of nondependent lung airways and allow some oxygen distention of the gas-exchanging alveolar space in the nondependent lung ( Fig. 49-30C ) without significantly affecting the pulmonary vasculature. In all clinical studies,[385] [388] [389] [392] the application of 5 to 10 cm H2 O CPAP has not interfered with the performance of surgery and may, in fact, facilitate intralobar dissection. This finding is not surprising in view of the fact that the initial compliance of a collapsed lung is only 10 mL/cm H2 O, and 5 to 10 cm H2 O CPAP should create only a slightly distended lung that occupies a volume of 50 to 100 mL, which is hardly or not at all noticed by the surgeon.
On the other hand, in a canine study,[391] 15 cm H2 O of nondependent lung CPAP caused changes in PaO2 and shunting similar to those of 5 to 10 cm H2 O of nondependent lung CPAP, whereas blood flow to the nonventilated nondependent lung decreased significantly. Therefore, high levels of nonventilated lung CPAP act by permitting oxygen uptake in the nonventilated lung, as well as by causing diversion of blood flow to the ventilated lung, where both oxygen and carbon dioxide exchange can take place (see Fig. 49-30C ). Because low levels of nonventilated lung CPAP are as efficacious as high levels and have less surgical interference and hemodynamic implications, it is logical to use low levels of nonventilated CPAP first.
In all patients in all clinical studies to date, 5 to 10 cm H2 O of nondependent lung CPAP has significantly increased PaO2 during one-lung ventilation.[385] [388] [389] [392] [393] [394] [395] It should be concluded that the single most efficacious maneuver to increase PaO2 during one-lung ventilation is to apply 5 to 10 cm H2 O of CPAP to the nondependent lung. In our experience, low levels of nonventilated lung CPAP have corrected severe hypoxemia (PaO2 <50 mm Hg) more than 95% of the time, provided that the DLT was correctly positioned. However, the nondependent lung CPAP must be applied during the deflation phase of a large tidal volume so that the deflating lung can lock into a CPAP level with uniform expansion and obviate the need to overcome the critical opening pressure of airways and alveoli.
In both human[388] and canine [391] studies, oxygen insufflation at zero airway pressure did not significantly improve PaO2 and shunting, and this result was probably due to the inability of zero transbronchial airway pressure to maintain airway patency and overcome critical alveolar opening pressure. Although one study in patients has concluded that insufflation of O2 at zero airway pressure does increase PaO2 , the study is difficult to interpret because the patients did not serve as their own controls.[396]
Several selective nondependent lung CPAP systems that are easy to assemble have been described.[389] [393] [394] [395]
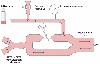 Figure 49-31
The three essential components of a nondependent lung
continuous positive airway pressure (CPAP) system consist of (1) an oxygen source,
(2) a pressure relief valve, and (3) a pressure manometer to measure CPAP. CPAP
is created by the free flow of oxygen into the lung versus the restricted outflow
of oxygen from the lung by the pressure relief valve. PEEP, positive end-expiratory
pressure; ZEEP, zero end-expiratory pressure. (From Benumof JL: Anesthesia
for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-31
The three essential components of a nondependent lung
continuous positive airway pressure (CPAP) system consist of (1) an oxygen source,
(2) a pressure relief valve, and (3) a pressure manometer to measure CPAP. CPAP
is created by the free flow of oxygen into the lung versus the restricted outflow
of oxygen from the lung by the pressure relief valve. PEEP, positive end-expiratory
pressure; ZEEP, zero end-expiratory pressure. (From Benumof JL: Anesthesia
for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
The availability of a commercial nondependent nonventilated lung CPAP device (Mallinckrodt Medical, Inc.) has replaced the need for all homemade devices. The Broncho-Cath CPAP system ( Fig. 49-32 ) is similar in concept to the system shown in Figure 49-31 , except that the restrictive mechanism is a continuously vented slot (opening); the larger the vent slot, the lower the CPAP level delivered, and the smaller the vent slot, the higher the CPAP level. The level of CPAP is selected by simply turning an outer calibrated cylinder (calibrated for an oxygen flow rate of 5 L/min), which progressively uncovers the inner (pop-off) slot. Because the dial is calibrated and marked with the CPAP level selected, a pressure manometer is unnecessary. The rest of the CPAP system consists of the other components shown in Figure 49-31 (oxygen tubing, reservoir bag). A unique feature of the Broncho-Cath CPAP system is the fact that the valve is continuously vented. Because the vent slot cannot be totally occluded, the danger of overpressurization of the nondependent lung is minimized. In addition, the CPAP system is calibrated from 1 to 10 cm H2 O pressure, which permits accurate delivery of very low levels of CPAP. This may be desirable with extremely compliant lungs or when maximum surgical exposure is required. This small, lightweight CPAP device can be included within the DLT package (the DLT is available with or without the CPAP device), is extremely easy to use, and is preassembled, cost-effective, sterile, reliable, and disposable. Perhaps its most important attribute is that a preassembled device is obviously more readily available than devices that require a search for spare parts, and the commercial product has built-in quality assurance that cannot be duplicated by individual remedies.
In theory and from the preceding considerations, it appears that
the ideal way to improve oxygenation during one-lung ventilation is the application
of differential lung PEEP/CPAP (see Fig.
49-30D
) (see the following section for the step-by-step approach). In
this situation, the ventilated (dependent) lung is given PEEP in the usual conventional
manner in an effort to improve ventilated lung volume and V̇/![]() relationships.
Simultaneously, the nonventilated (nondependent) lung receives CPAP in an attempt
to improve oxygenation of the blood perfusing this lung. Therefore, with differential
lung PEEP/CPAP, it does not matter where the blood flow goes nearly as much as during
simple one-lung ventilation because wherever it goes (to either ventilated or nonventilated
lung), it has at least some chance to participate in gas exchange with alveoli that
are expanded with oxygen. In indirect support of this contention, arterial oxygenation
relationships.
Simultaneously, the nonventilated (nondependent) lung receives CPAP in an attempt
to improve oxygenation of the blood perfusing this lung. Therefore, with differential
lung PEEP/CPAP, it does not matter where the blood flow goes nearly as much as during
simple one-lung ventilation because wherever it goes (to either ventilated or nonventilated
lung), it has at least some chance to participate in gas exchange with alveoli that
are expanded with oxygen. In indirect support of this contention, arterial oxygenation
 Figure 49-32
The Mallinckrodt Broncho-Cath continuous positive airway
pressure (CPAP) system. A, Photograph of the entire
CPAP system connected to a double-lumen tube. B,
Schematic of the entire CPAP system. C, Close-up
of the CPAP-generating restrictive mechanism. (Courtesy of Mallinckrodt
Medical, Inc., St Louis.)
Figure 49-32
The Mallinckrodt Broncho-Cath continuous positive airway
pressure (CPAP) system. A, Photograph of the entire
CPAP system connected to a double-lumen tube. B,
Schematic of the entire CPAP system. C, Close-up
of the CPAP-generating restrictive mechanism. (Courtesy of Mallinckrodt
Medical, Inc., St Louis.)
Multiple studies have reported significant increases in oxygenation with the application of differential lung ventilation and PEEP (either PEEP/PEEP, PEEP/CPAP, or CPAP/CPAP) through DLTs to intensive care unit patients with acute respiratory failure secondary to predominantly unilateral lung disease.[397] In all cases, conventional two-lung therapy (mechanical ventilation, PEEP, CPAP) had been administered through a standard single-lumen tube and either failed to improve or actually decreased oxygenation. In these patients the single-lumen tube was replaced with a DLT. In most cases, the amount of PEEP initially administered to each lung was inversely proportional to the compliance of each lung; ideally, this PEEP arrangement should result in equal FRC in each lung. In some cases, the amount of PEEP that each lung received was later adjusted and titrated in an effort to find a differential lung PEEP combination that resulted in the lowest right-to-left transpulmonary shunt.
Figure 49-33 summarizes the recommended plan for obtaining satisfactory arterial oxygenation during one-lung anesthesia. Two-lung ventilation is maintained for as long as possible (usually until the pleura is opened). When one-lung ventilation is commenced, a tidal volume of 10 mL/kg is used, and the respiratory rate is adjusted so that PaCO2 equals 40 mm Hg. A high inspired oxygen concentration (FIO2 of 0.8 to 1.0) should be used, and SaO2 should be monitored continuously.
If hypoxemia is present after this initial conventional approach, two major causes of hypoxemia, namely, malposition of the DLT and poor hemodynamic status, must be ruled out. Proper tube position should be confirmed with fiberoptic bronchoscopy. If the DLT is correctly positioned and the patient's hemodynamic status is satisfactory, simple tidal volume and respiratory rate adjustments should be made. [302] For example, if the tidal ventilation is thought to be too high, it should be decreased, and if the tidal ventilation is thought to be too low, it should be increased. If these simple maneuvers do not quickly resolve the problem, the studies of selective nondependent lung
 Figure 49-33
An overall one-lung ventilation plan. ASAP, as soon
as possible; CPAP, continuous positive airway pressure; FIO2
,
inspired oxygen concentration; PEEP, positive end-expiratory pressure; RR, respiratory
rate; TV, tidal volume.
Figure 49-33
An overall one-lung ventilation plan. ASAP, as soon
as possible; CPAP, continuous positive airway pressure; FIO2
,
inspired oxygen concentration; PEEP, positive end-expiratory pressure; RR, respiratory
rate; TV, tidal volume.
If severe hypoxemia persists after the application of differential
lung PEEP/CPAP (which would be extremely rare), it should be remembered that the
nondependent lung may be intermittently ventilated with positive pressure with oxygen
(see Fig. 49-33
). Finally,
most of the V̇/![]() imbalance is eliminated during pneumonectomy by tightening
a ligature around the nonventilated lung pulmonary artery as early as possible, thus
directly eliminating all shunt flow through the nonventilated lung (see Fig.
49-33
). Indeed, clamping the pulmonary artery to a collapsed lung functionally
resects the entire lung, and PaO2
is restored
to a level not significantly different from a two-lung ventilation or postpneumonectomy
one-lung ventilation value.
imbalance is eliminated during pneumonectomy by tightening
a ligature around the nonventilated lung pulmonary artery as early as possible, thus
directly eliminating all shunt flow through the nonventilated lung (see Fig.
49-33
). Indeed, clamping the pulmonary artery to a collapsed lung functionally
resects the entire lung, and PaO2
is restored
to a level not significantly different from a two-lung ventilation or postpneumonectomy
one-lung ventilation value.
Because nondependent lung CPAP has been shown to relieve hypoxemia consistently and reliably during one-lung ventilation,[388] [389] its routine use to prevent
|
|
|
|
|
|
|
|
|
|
|
|
|