|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thoracic surgery may be greatly facilitated by causing selective atelectasis of the lung being operated on (one-lung ventilation/anesthesia conditions). The normal response of the pulmonary vasculature to atelectasis is an increase in PVR (in just the atelectatic lung). The mechanism of the increase in PVR is partly mechanical, but to a great extent it is also due to hypoxic pulmonary vasoconstriction (HPV).[153] [154] [155] The selective increase in atelectatic lung PVR diverts blood flow from the atelectatic lung toward the remaining normoxic or hyperoxic ventilated lung. Figure 49-6 shows the theoretically expected effect of HPV on PaO2 as the amount of lung that is made hypoxic increases. When the percentage of lung that is hypoxic is between 30% and 70%, which encompasses the one-lung ventilation/anesthesia condition, there is a large difference between the PaO2 expected with a normal amount of HPV and that when there is no HPV. In fact, in this range of hypoxic lung, HPV increases PaO2 from levels that might cause arrhythmias to much higher and safer values. Thus, HPV is an autoregulatory mechanism that protects PaO2 by decreasing the amount of shunt flow that can occur through hypoxic lung.
General anesthesia with controlled ventilation is the safest method of anesthetizing patients for the vast majority of elective thoracic procedures. Inhibition of HPV in the nonventilated nondependent lung by the anesthetic should be prevented. All the inhaled and many of the injectable anesthetics have been studied with regard to their effect on HPV. Halothane was initially studied the most intensly. [155] [156] [157] [158] [159] [160] [161] [162] [163] [164] [165] [166] [167] [168] [169] [170] [171] [172] [173] [174] [175] [176] [177] In vitro studies demonstrated significant HPV inhibition; however, intact animals demonstrated far less. Important methodologic differences between in vitro and in vivo-not intact preparations and in vivo-intact and human models that could account for the observed differences in halothane's
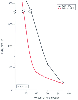 Figure 49-6
Effect of hypoxic pulmonary vasoconstriction (HPV) on
PaO2
. As the amount of lung that is made
hypoxic is increased (x axis), arterial oxygen tension
(PaO2
) decreases (y
axis). In the range of 30% to 70% hypoxic lung, the normal expected amount of HPV
increases PaO2
from arrhythmogenic levels
to much higher and safer levels. Normal cardiac output, hemoglobin concentration,
and mixed venous oxygen tension (Pv̄O2
)
are assumed. (Data from Marshall BE, Marshall C: Continuity of response
to hypoxic pulmonary vasoconstriction. J Appl Physiol 59:189, 1980.)
Figure 49-6
Effect of hypoxic pulmonary vasoconstriction (HPV) on
PaO2
. As the amount of lung that is made
hypoxic is increased (x axis), arterial oxygen tension
(PaO2
) decreases (y
axis). In the range of 30% to 70% hypoxic lung, the normal expected amount of HPV
increases PaO2
from arrhythmogenic levels
to much higher and safer levels. Normal cardiac output, hemoglobin concentration,
and mixed venous oxygen tension (Pv̄O2
)
are assumed. (Data from Marshall BE, Marshall C: Continuity of response
to hypoxic pulmonary vasoconstriction. J Appl Physiol 59:189, 1980.)
However, the most likely explanation is that the intact animal preparations possess some biologic or physiologic property that greatly lessens the inhibitory effect of anesthetics on HPV. Indeed, older studies of halogenated drugs other than halothane, namely, isoflurane,[184] [185] [186] [187] [188] [189] [190] [191] enflurane,[192] [193] [194] and methoxyflurane,[195] [196] demonstrated dose-related inhibition of HPV (especially with in vitro preparations). Nitrous oxide exerts a minor, clinically insignificant inhibition of HPV.[197] [198] [199] Similarly, intravenous anesthetics cause minimal inhibition of HPV.[200] [201] [202] [203] [204] [205]
Anesthetic drugs can (minimally) impair arterial oxygenation during one-lung anesthesia by inhibiting HPV in the nonventilated lung. One study investigating the effect of isoflurane on regional canine HPV was especially well controlled and showed that when all nonanesthetic drug variables that might change regional HPV are kept constant, isoflurane inhibits single-lung HPV in a dose-dependent manner. [186] This study also demonstrated the relationship between the dose of isoflurane administered and the degree of inhibition of the single-lung canine HPV response. To provide a clinical perspective of the HPV response it is first necessary to understand what should happen to blood flow, shunt flow, and arterial oxygenation as a function of a normal amount of HPV when two-lung ventilation is changed to one-lung ventilation in the LDP. Once the stable one-lung ventilation condition has been described, it is possible, by using data from the previously mentioned study, to see how isoflurane administration would affect the one-lung ventilation blood flow distribution, shunt flow, and arterial oxygen tension (PaO2 ).
Gravity causes a vertical gradient in the distribution of pulmonary blood flow in the LDP for the same reason that it does in the upright position. Consequently, blood flow to the dependent lung is significantly greater than blood flow to the nondependent lung. When the right lung is nondependent, it should receive approximately 45% of total blood flow as opposed to the 55% that it received in the upright and supine positions. When the left lung is nondependent, it should receive approximately 35% of total blood flow as opposed to the 45% that it received in the upright and supine positions (closed-chest data with normal pulmonary artery pressure). [206] [207] [208] If these blood flow distributions are combined (both the right and left lungs being nondependent an equal number of times), the average two-lung ventilation blood flow distribution in the LDP would consist of 40% of total blood flow perfusing the nondependent lung and 60% of total blood flow perfusing the dependent lung ( Fig. 49-7, left-hand panel ).
When the nondependent lung is nonventilated (made atelectatic), HPV in this lung will increase its PVR and decrease its blood flow. In the absence of any confounding or inhibiting factors to the HPV response, a single-lung HPV response should decrease the blood flow to that lung by 50%.[209] Consequently, the nondependent lung should be able to reduce its blood flow from 40% to 20% of total blood flow, and the nondependent-dependent lung blood flow ratio during one-lung ventilation should be 20:80 (see Fig. 49-7, middle panel ).
All the blood flow to the nonventilated nondependent lung is shunt flow, and therefore one-lung ventilation creates an obligatory right-to-left transpulmonary shunt flow that was not present during two-lung ventilation.
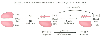 Figure 49-7
Effect of 1 minimum alveolar concentration (MAC) isoflurane
anesthesia on shunt during one-lung ventilation (1LV) of normal lungs. This diagram
shows that for two-lung ventilation, the ratio of the percentages of blood flow to
the nondependent and dependent lungs is 40:60 (left-hand side).
When two-lung ventilation is converted to one-lung ventilation (as indicated by
atelectasis of the nondependent lung), the hypoxic pulmonary vasoconstriction (HPV)
response decreases blood flow to the nondependent lung by 50%, so the nondependent-dependent
lung blood flow ratio is now 20:80 (middle). According
to the data of Domino and colleagues,[186]
administration
of 1 MAC isoflurane anesthesia should cause a 21% decrease in the HPV response, which
would decrease the blood flow reduction from 50% to 40%. Consequently, the nondependent-dependent
lung blood flow ratio would now become 24:76, which represents a 4% increase in total
shunt across the lungs (right-hand side). (From
Benumof JL: Isoflurane anesthesia and arterial oxygenation during one-lung ventilation.
Anesthesiology 64:419, 1986.)
Figure 49-7
Effect of 1 minimum alveolar concentration (MAC) isoflurane
anesthesia on shunt during one-lung ventilation (1LV) of normal lungs. This diagram
shows that for two-lung ventilation, the ratio of the percentages of blood flow to
the nondependent and dependent lungs is 40:60 (left-hand side).
When two-lung ventilation is converted to one-lung ventilation (as indicated by
atelectasis of the nondependent lung), the hypoxic pulmonary vasoconstriction (HPV)
response decreases blood flow to the nondependent lung by 50%, so the nondependent-dependent
lung blood flow ratio is now 20:80 (middle). According
to the data of Domino and colleagues,[186]
administration
of 1 MAC isoflurane anesthesia should cause a 21% decrease in the HPV response, which
would decrease the blood flow reduction from 50% to 40%. Consequently, the nondependent-dependent
lung blood flow ratio would now become 24:76, which represents a 4% increase in total
shunt across the lungs (right-hand side). (From
Benumof JL: Isoflurane anesthesia and arterial oxygenation during one-lung ventilation.
Anesthesiology 64:419, 1986.)
Domino and coworkers[186] found the percent inhibition of the regional HPV response to equal 22.8 (percent alveolar isoflurane) minus 5.3 (see Fig. 49-7, top equation ).[186] The top equation and right-hand panel of Figure 49-7 show that isoflurane anesthesia at 1 minimum alveolar concentration (1 MAC) would inhibit the nondependent lung HPV response by approximately 21%, which would decrease this response from a 50% to a 40% blood flow reduction in this lung; in turn, nondependent lung blood flow would be increased from 20% to 24% of total blood flow, which would cause the shunt to increase by 4% of the cardiac output and PaO2 to decrease a moderate amount to 205 mm Hg (FIO2 = 1.0). A decrease in PaO2 and an increase in shunt of this magnitude are small and may not be detectable given the usual accuracy of clinical methodology. In fact, in clinical one-lung ventilation studies involving intravenously anesthetized patients with this level of shunting, administration of 1 MAC isoflurane (and halothane) anesthesia during stable one-lung ventilation conditions causes no detectable decrease in PaO2 . [177] [212] In one of these clinical studies,[212] stable one-lung ventilation conditions in the LDP were established in patients who were anesthetized with only intravenous drugs. While stable one-lung ventilation was maintained, inhaled anesthetics were administered (halothane and isoflurane end-tidal concentrations were greater than 1 MAC for at least 15 minutes) and then discontinued (halothane and isoflurane end-tidal concentrations decreased to near zero). In the other study, [177] steady-state one-lung ventilation conditions in the LDP were established in patients who were anesthetized with only inhaled drugs (halothane and isoflurane end-tidal concentrations were greater than 1 MAC for more than 40 minutes). While one-lung ventilation was continued, inhaled anesthesia was then discontinued and intravenous anesthesia administered (halothane and isoflurane end-tidal concentrations decreased to near 0). There was no significant difference in PaO2 during inhaled anesthesia with either halothane or isoflurane when compared with intravenous anesthesia during one-lung ventilation in either of the two experimental sequences. In addition, there were no significant changes in physiologic variables, such as cardiac output, PVR, and Pv̄O2 , that might secondarily alter nondependent lung HPV. Thus, irrespective of whether inhaled anesthesia is administered before or after intravenous anesthesia during one-lung ventilation, inhaled anesthesia does not further impair arterial oxygenation. Additionally, a recent study comparing oxygenation and shunt fraction during one-lung ventilation demonstrated no significant difference between inhaled sevoflurane and isoflurane.[213] These findings are consistent with the interpretation that 1 MAC halothane or isoflurane in patients with a moderate level of shunting does not inhibit HPV enough to cause a significant decrease in PaO2 during one-lung ventilation in the LDP. Almost identical results have been obtained during one-lung ventilation with nitrogen (while the other lung was ventilated with 100% oxygen) in volunteers who were alternately anesthetized with isoflurane and intravenous drugs[191] and enflurane and intravenous drugs. [193]
General anesthesia with controlled ventilation (see Chapter 5 , Chapter 6 , Chapter 7 , Chapter 8 , Chapter 9 , Chapter 10 , Chapter 11 , Chapter 12 , and Chapter 13 ) is the safest method of anesthetizing patients for the vast majority of elective thoracic procedures. Although a variety of general anesthesia techniques can be used, the volatile halogenated anesthetic drugs are good choices for several reasons. First, the halogenated drugs have a salutary effect on airway irritability. The mechanism of this action is controversial, but as previously discussed, there is evidence that these drugs can block specific forms of bronchoconstriction,[214] [215] as well as have a nonspecific bronchodilating effect related to the depth of anesthesia.[215] Obtundation of airway reflexes in patients who have reactive airways (i.e., smokers) and who may have their airways directly manipulated by the surgeon is a highly desirable property of the general anesthesia produced by these drugs. Second, the use of volatile halogenated drugs allows delivery of a high inspired oxygen concentration without loss of anesthesia. Although a nitrous oxide-oxygen-narcotic-relaxant anesthesia technique can be used, nitrous oxide necessitates a significant decrease in FIO2 and increases the chance of development of hypoxemia (especially if one-lung ventilation is used).[216] Unless very high doses of narcotics are used, airway reflexes and reactivity may remain at a high level. Third, because the volatile halogenated drugs can be rapidly eliminated, concern about postoperative hypoventilation in extubated patients may be diminished. Relatively high doses of intravenous anesthetics, such as narcotics, ketamine, and barbiturates, may cause the patient to require a period of postoperative ventilation. Fourth, in the usual clinical doses (near 1 MAC), halogenated anesthetic drugs provide a reasonable degree of cardiovascular stability, which may be of particular importance in patients who have a history of smoking and therefore a high incidence of coronary artery disease and systemic hypertension.[217] [218] Fifth, the halogenated drugs do not appear to decrease PaO2 any more than intravenous anesthetics do during one-lung ventilation (see the following section).[177] [212]
The narcotics, especially fentanyl, have a number of desirable properties (see Chapter 10 and Chapter 11 ) that could be used to advantage in patients undergoing thoracic surgery. First, fentanyl has no significant adverse hemodynamic effects and is therefore a useful drug in patients who have coronary artery disease. Second, if significant blood levels exist at the end of surgery, the narcotics can allow an intubated patient to have a smooth transition from surgery to the postoperative period. Third, if used in moderate dosage, narcotics diminish the amount of volatile halogenated anesthetic required to achieve surgical levels of anesthesia. Fourth, high doses of narcotics or moderate doses in conjunction with halogenated drugs allow the use of high FIO2 without loss of anesthesia. Fifth, narcotics are thought to not diminish regional HPV and should therefore permit optimal oxygenation during one-lung ventilation.
Ketamine in combination with nitrous oxide and a muscle relaxant has also been used as anesthesia for thoracic surgery.[219] Although we do not ordinarily use ketamine for elective thoracic procedures, the drug is useful for induction of general anesthesia in critically ill patients undergoing emergency thoracic surgery for several reasons. First, ketamine has sympathomimetic properties[220] that are highly desirable because many emergency thoracic procedures are associated with hypovolemia (gunshot and stab wounds to the chest, blunt trauma, and massive hemoptysis). However, it should be remembered that ketamine depresses cardiovascular function (systemic blood pressure, cardiac contractility) if the degree of hypovolemia is severe and the patient is sympathetically exhausted. Second, ketamine has a rapid onset of action and can be used safely, along with cricoid pressure, to induce anesthesia in patients with full stomachs. Third, ketamine may reduce bronchospasm in asthmatic patients[221] ; clinical extrapolation of this effect to thoracic surgical patients is uncertain but seemingly reasonable at this time. Fourth, ketamine does not impair arterial oxygenation during one-lung ventilation (perhaps because of its lack of effect on HPV).[204]
There are benefits to using predominantly an inhaled and intravenous anesthetic technique intraoperatively. Although most now agree that postoperative analgesia is best with a preoperatively placed thoracic epidural block, the intraoperative use of epidural blockade has pros and cons. One clinical study made this point very well by examining the respiratory and cardiac differences between various anesthetic regimens (intravenous based, inhalation based, epidural based) before and during one-lung ventilation.[222] The 36 patients were randomly allocated to one of the following groups: A—propofol, 10 mg/kg/hr, fentanyl; B—1 MAC enflurane, fentanyl; and C—thoracic epidural anesthesia, 0.4% enflurane. Before induction of anesthesia, significant differences were not found. During two-lung ventilation, the cardiac index was significantly decreased in the B group in comparison to the C group (P = .01). During one-lung ventilation, significant differences were not found except for an increased shunt fraction (QS/QT) in group B (A versus B, P = .05; B versus C, P = .05; A versus C, not significant). Because QS/QT was significantly increased and hypoxemia occurred with regimen B, regimen A or C might be preferred in patients at high risk for hypoxia or in situations in which the application of CPAP to the nondependent lung is not possible. The cardiac index was best maintained in group C. Regimen C might be of value in patients at high risk for poor perfusion if one takes into account both the possible complications of the epidural block and the reduced need for postoperative analgesics.
The following recommended anesthetic technique takes advantage of the desirable properties and minimizes the undesirable properties of these drugs. Thus, halogenated drugs are used for their effect on bronchomotor tone, to allow administration of 100% oxygen, and to permit early extubation without decreasing hemodynamic function and arterial oxygenation; fentanyl is used to ensure hemodynamic stability without jeopardizing early extubation if desired. If it is thought that the patient will
The reader will find tips on placement of a thoracic epidural catheter in the later section "Management of Postoperative Pain." Important management factors include the following: (1) perform a neurologic examination on the patient before initiating placement of the epidural catheter, (2) place the thoracic epidural catheter only in an awake patient preoperatively, (3) document the efficacy of catheter placement by obtaining a band of anesthesia over the operative site with a test dose of local anesthetic, (4) demonstrate and document that the patient has the same neurologic examination (particularly in regard to motor function) that was documented before catheter placement, and (5) dose the catheter with an opiate (we prefer hydromorphone [Dilaudid]) and decide whether the patient's hemodynamic status is robust enough to tolerate a concomitant local anesthetic for the procedure (if not, the local anesthetic can be added as an infusion [combined with an opiate] at the end of the case).
The patient is preoxygenated by spontaneously breathing 100% oxygen through an anesthesia mask that is connected to an anesthesia circle system. Fentanyl is administered intravenously until the respiratory rate is approximately 8 to 10 breaths/min. This rate usually corresponds to a dose of 3 to 6 µg/kg and is generally administered over a period of several minutes. When the respiratory rate is relatively slow and deep and response to commands is becoming sluggish, a small dose of sodium thiopental (2 to 3 mg/kg), ketamine (1.0 to 2.0 mg/kg—if the patient is thought to have an especially reactive airway), or etomidate (0.1 to 0.2 mg/kg—if the patient is thought to be moderately hypovolemic or have impaired cardiovascular status) is administered to render the patient unconscious and usually apneic. Control of the airway is then established and ventilation is begun with intermittent positive-pressure oxygen through the mask. A nondepolarizing neuromuscular blockade drug can be administered at this point. While the patient is being ventilated with positive pressure, a concentration of 1.0% to 3.0% sevoflurane is administered. The higher sevoflurane concentration is used initially for a short time (overpressure, 1 to 2 minutes); as the patient demonstrates signs of deepening anesthesia, the inspired sevoflurane concentration is decreased. In view of the fact that general anesthetics significantly decrease the ventilatory response to carbon dioxide (to a much greater degree in patients with mechanical ventilatory impairment than in normal patients), patients are not allowed to breathe spontaneously until the end of the procedure because alarming degrees of hypercapnia have been observed in similar circumstances when spontaneous ventilation was allowed.[223]
The development of full paralysis, as a result of the previously administered drug, is noted with a neuromuscular blockade monitor. During the period of deepening sevoflurane anesthesia and paralysis, blood pressure is supported with small doses of vasopressors, if needed, because crystalloid infusion is minimized in patients undergoing thoracotomy unless bleeding is encountered intraoperatively. When the patient has been judged to have been adequately (surgical stage) anesthetized (as ascertained by changes in blood pressure, heart rate, and eye signs [the eyes should be central, conjugate, fixed, staring, without tears, and with nondilated pupils]) and paralyzed in this manner, laryngoscopy is performed, the tracheobronchial tree is sprayed with a laryngotracheobronchial topical anesthesia spray system (or intravenous lidocaine may be administered), and the trachea is intubated with a double-lumen tube (or a single-lumen tube if a bronchial blocker will be used for lung separation). The intravenous or intratracheal lidocaine (or both) will diminish both the airway and cardiovascular response to endotracheal intubation.[224] However, patients who have received fentanyl (3 to 6 µg/kg) on induction rarely manifest a minimal response to laryngoscopy after the aforementioned induction sequence. The patient is then ventilated with maintenance doses of isoflurane and given maintenance doses of narcotics and relaxants. Use of maintenance paralysis decreases isoflurane requirements, possibly allowing for more rapid emergence from anesthesia.
Anesthesia is maintained with either sevoflurane or isoflurane (concentration of approximately 0.5 to 1.0 MAC) and the thoracic epidural, and subsequent intravenous narcotics are avoided. This technique is used primarily if the patient is thought to have a reasonable chance of being extubated at the end of the procedure. If the patient is thought to not have a reasonable chance of being extubated in the immediate postoperative period and to require a significant period of postoperative ventilation, more liberal use of intravenous narcotics is appropriate, and the thoracic epidural can be reserved for later use. Relaxants are administered in small doses to keep the level of neuromuscular blockade, as judged by a neuromuscular blockade monitor, near the 90% paralysis level.
The goal, at the end of the procedure, is to have complete train of four and no fade with tetanus if the patient is to be extubated in the operating room. If the patient is not expected to be ready for extubation of the trachea in the first postoperative hour, additional neuromuscular blockade is administered, the patient is turned supine and placed back on 100% oxygen, the anesthetic level is ensured, the double-lumen tube is changed to a single-lumen tube and the position verified with end-tidal CO2 monitoring, and positive-pressure ventilation is resumed.
|
|
|
|
|
|
|
|
|
|
|
|
|