|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Monitoring requirements (see Chapter 30 , Chapter 31 , Chapter 32 , Chapter 33 , Chapter 34 , Chapter 35 , and Chapter 36 ) differ among individual patients for two reasons. First, patients undergoing thoracic operations have varying degrees of preexisting cardiorespiratory disease. Second, the very nature of thoracic procedures (e.g., one-lung ventilation) causes further derangements in respiratory and cardiovascular function in the perioperative period. On the basis of these two considerations and their interactions, individual patients can and should be categorized into a progressively sophisticated and complex tier system with regard to what monitoring is necessary during anesthesia to make possible accurate and rapid diagnosis and therapy. It should be apparent from this monitoring approach that an individual with severe pulmonary disease who is to undergo a minor surgical procedure may well require as extensive a monitoring system as a patient with normal lungs who is to have extensive thoracic surgery.
Table 49-9 presents three major categories of patients undergoing thoracic surgery and the monitoring recommended for each category. The first category (tier I) includes healthy patients without special intraoperative conditions, such as a young patient undergoing pleurodesis. This tier contains the minimal, yet essential monitoring required for any patient undergoing a thoracic procedure.
Because failure to check the equipment properly before induction of anesthesia is responsible for 22% of the critical incidents that occur during anesthesia,[143] the considerations discussed in Table 49-9 presume that the function of the anesthesia machine and ventilator is assessed preoperatively in an orderly and complete fashion. Failure of oxygen delivery to the patient must be signaled by audiovisual alarms from an inspired oxygen monitor. Pulse oximetry will also serve as a relatively late indicator of an inappropriately decreased inspired oxygen fraction (FIO2 ). Observation of the respiratory rate and movement of the rebreathing bag, ventilator bellows, and chest wall permits a rough estimation of minute ventilation and, along with constant use of a stethoscope to hear breath sounds, provides for rapid diagnosis of apnea or disconnection of a breathing circuit. However, end-tidal CO2 (PETCO2 ) monitoring allows precise breath-by-breath analysis and diagnosis of all these respiratory functions (apnea, minute ventilation, gas exchange) and is therefore now an essential monitor. In addition, because the slope of the alveolar plateau correlates positively with airway resistance, PETCO2 can function as a rough continuous monitor of airway mechanics. The stethoscope is also used to assess airway mechanics (detection of bronchospasm), along with an "educated" hand on the rebreathing bag (manual detection of total lung compliance and resistance). Adequacy of gas exchange is further assessed by observing the color of shed blood, looking for cyanosis (nail beds, lips, mucous membranes, skin), and listening to the character of the breath sounds. More importantly, pulse oximetry allows precise, on-line, beat-by-beat, continuous monitoring of arterial oxygenation and is therefore now an essential monitor. Pulse oximetry also indicates desaturation when blood pressure or blood flow is very low and is therefore an on-line monitor of severe changes in cardiovascular function. Because pulse oximetry is a crude monitor in this regard, cardiovascular function must be monitored by frequent use of the blood pressure cuff or, increasingly commonly, an automated oscillometric blood pressure monitor and by continuous ECG. With constant minute ventilation, acute changes in PETCO2 are proportional to acute changes in cardiac output (i.e., pulmonary blood flow). Muscle relaxation is assessed by simple observation for motor activity and by a peripheral nerve stimulator. Body temperature is continuously measured by a probe. These recommendations for monitoring are routine during anesthesia for nearly all types of surgery.
It should be emphasized that if a serious question arises about the adequacy of arterial oxygenation or minute ventilation (or both) while the pulse oximeter and PETCO2 monitor, respectively, are being used, blood should be drawn for determination of arterial blood gases (see tier II); thus, pulse oximetry and PETCO2 monitoring should not be regarded as a total substitute for arterial blood gas studies. However, venous blood from the back of a warmed hand or a large neck vein obtained with no tourniquet or a short-time low-pressure tourniquet has a PCO2 close enough to that of arterial blood (usually 4 to 8 mm Hg) to be useful for most clinical purposes.[144] In addition, under these sampling conditions, the presence of a sufficiently high oxygen tension and saturation of venous blood (>40 mm Hg and 75%, respectively) provides a good indication that arterial hypoxemia is most probably absent. As an alternative sampling site, capillary earlobe blood (obtained by needle prick or cut) is usually acceptable for clinical management because this blood has a PaCO2 that is within 2.0% of simultaneous arterial samples.[145]
The second tier in Table 49-9 represents an increase in risk caused by either special unfavorable intraoperative conditions in relatively healthy patients or significant preexisting cardiopulmonary disease in patients who will not experience special intraoperative conditions. An example of the former circumstance is a patient with mild lung disease who is having a lobectomy. An example of the latter circumstance is a patient with moderate to severe interstitial lung disease who requires an open lung biopsy. Anyone with some lung disease undergoing one-lung ventilation for a major thoracic procedure must be considered to be in a tier II category. For this second monitoring level, in addition to the preceding essential system (tier I), the following monitoring modalities are required: a respirometer to permit accurate measurement of tidal volume and minute ventilation (the ventilator bellows can be very inaccurate), arterial blood gas analysis to ensure adequate ventilation and oxygenation, an inspiratory pressure gauge to calculate and monitor changes in whole-lung and individual-lung dynamic and
| Tiered System | Patient Category | Gas Exchange | Airway Mechanics | Endotracheal Tube Position | PA Pressures | Cardiovascular Status |
|---|---|---|---|---|---|---|
|
Tier I: Essential monitors used in all patients * |
Routine healthy patients without special intraoperative conditions | Color of tissues and shed blood SpO2 PETCO2 | Feel of the breathing bag, stethoscope, PIP, PETCO2 | EBBS (except ipsilateral tube clamp because ipsilateral breath sounds disappear). Ballotable balloon in SSN, FOB after placed in LDP | Not measured | NIBP, pulse oximeter waveform, ECG, PETCO2 , esophageal stethoscope, ± CVP, ± invasive arterial pressure monitoring |
|
Tier II: Special intermittent or continuous monitoring needs |
Healthy patients with special procedures or sick patients with routine procedures | As above plus frequent ABG studies | As above plus spirometry. Individual- and whole-lung compliance | FOB to verify tube position while in supine position, as well as in the LDP | Measure Ppa if lobectomy or lung resection | As above, plus invasive arterial pressure monitoring, + CVP, + PA catheter (if poor EF, PA, HTN), ± TEE |
|
Tier III: Advanced monitoring |
Sick patients with special intraoperative conditions | As above plus QS/QT, VD/VT, frequent VBGs | As above plus airway resistance | As above plus frequent rechecks to verify position | Measure PA, Q, PVR, SVR, DaO2 -DvO2 | As above plus PA + TEE |
| ABG, arterial blood gas; CVP, central venous pressure; DaO2 -DvO2 , arterial venous content difference (the amount consumed by tissue metabolism; EBBS, equal bilateral breath sounds; ECG, electrocardiogram; EF, ejection fraction; FOB, fiberoptic bronchoscope; HTN, hypertension; LDP, lateral decubitus position; NIBP, noninvasive blood pressure; PA, pulmonary artery; PETCO2 , partial pressure of end tidal CO2 ; PIP, peak inspiratory pressure; Ppa, pulmonary arterial pressure; PVR, pulmonary vascular resistance; Q, cardiac output; QS/QT, right-to-left transpulmonary shunt; SpO2 , saturation of oxygen measured by pulse oximetry; SSN, suprasternal notch; SVR, systemic vascular resistance; TEE, trans-esophageal echocardiography; VBGs, mixed venous blood gases; VD/VT, dead space-to-tidal volume ratio. | ||||||
Finally, a third tier of monitoring requirements is designed for patients with significant preexisting cardiopulmonary disease who will experience further compromising intraoperative conditions. An example of such a patient is one with cor pulmonale undergoing lobectomy or pneumonectomy. For this high-risk group of patients, the anesthesiologist should consider the following additional monitoring: adequacy of tissue oxygenation continuously assessed by in-line arterial or mixed venous PO2 and PCO2 ; measurement of mixed venous oxygen saturation (Sv̄O2 ), which is especially helpful as an index of global well-being in that a major decrease is probably never a good event (Sv̄O2 is decreased by either a reduction in cardiac output, an increase in oxygen consumption, or a decrease in arterial oxygen content [CaO2 ]); an esophageal ECG obtained by modifying an esophageal stethoscope and used to differentiate atrial arrhythmias[146] ; a V5 ECG lead to detect myocardial ischemia by analysis of the ST segment of the ECG, which is especially important in patients with coronary artery disease; and a pulmonary artery catheter with a thermistor to permit continuous measurement of intravascular filling pressure and rapid determination of cardiac output (and therefore myocardial work indices and assessment of myocardial contractility). Advanced monitoring mainly confined to research at this time includes measurement of right-to-left transpulmonary shunt, ventilation dead space, airway resistance, and aortic pulse pressure contour for beat-to-beat estimation of cardiac output and myocardial contractility.
Two of the preceding monitoring recommendations deserve special discussion and emphasis: arterial cannulation and pulmonary artery catheterization. Direct arterial cannulation (usually of the radial artery) permits frequent arterial blood gas analysis (and perhaps continuous in-line measurement of arterial PO2 and PCO2 ); such analysis alone is sufficient justification for placement of an arterial line in patients with double-lumen endotracheal tubes (DLTs) or serious respiratory disease (or both). In addition, an arterial line permits continuous measurement of systemic blood pressure; similarly, this function alone is frequently sufficient justification for placement of an arterial line in patients with serious cardiovascular compromise. Additionally, mean pressure can be plotted over time to allow more precise measurement of perfusion pressure. Finally, an increase in positive pressure-induced variation in systolic blood pressure may be an early indicator of hypovolemia.[147]
Normally, central venous pressure is an adequate index of intravascular volume status. With progressive pulmonary disease or LV dysfunction, or both, however, the left- and right-sided cardiac circulations must be assessed separately because central venous pressure may no longer reflect left-sided filling pressure (pulmonary artery wedge pressure [Ppaw]). In addition, with significant pulmonary disease or tachycardia, pulmonary artery diastolic pressure will be elevated above pulmonary artery wedge pressure, and a significant pulmonary artery diastolic-to-wedge pressure gradient will be present. In this situation, only the wedge pressure can be used to reflect left-sided cardiac volume/compliance status. Finally, cardiac output is easily determined by using pulmonary artery catheter thermodilution techniques. For these reasons, the use of a pulmonary artery rather than a central venous catheter should be considered if pulmonary hypertension or cor pulmonale (or both) and coronary artery disease are present, especially if extensive perioperative fluid shifts or blood loss is anticipated.
Pulmonary artery catheters usually (in >90% of cases) float to and are situated in the right lung.[148] Consequently, during right thoracotomy (left LDP), the pulmonary artery catheter will be in the nondependent lung and therefore either in a collapsed lung if one-lung ventilation is used or possibly in a zone 1 or 2 region of the lung if large-tidal volume two-lung ventilation is used. Conversely, when a left thoracotomy is performed (patient in the right LDP), the pulmonary artery catheter will be in the dependent lung and will probably be in a zone 3 region. Thus, it is theoretically possible that the pulmonary artery catheter might function differently or yield different pulmonary vascular pressure and cardiac output data during right versus left thoracotomy and during two-lung versus one-lung ventilation.
Indeed, with the pulmonary artery catheter tip located in the right lung, cardiac output is lower during right thoracotomy with one-lung ventilation (right lung collapsed) than during left thoracotomy with one-lung ventilation (left lung collapsed) in patients who were otherwise similar ( Fig. 49-5A .)[149] Consequently, it is possible that when the pulmonary artery catheter is located in the collapsed lung, where blood flow patterns may be distorted or the function of the thermistor interfered with (so that it is not free in the lumen of the vessel), the measured output is indeed lower. This hypothesis is supported by the concurrent finding that continuously measured Sv̄O2 is also decreased during right thoracotomy as compared with left thoracotomy when the pulmonary artery catheter is located in the collapsed nondependent lung. The decrease in Sv̄O2 may have been caused by stagnant blood flow and is therefore not truly representative of whole-patient Sv̄O2 .[149] When the nondependent lung is ventilated with varying levels of PEEP (in contrast to nondependent lung collapse), there is no difference in cardiac output measured simultaneously from thermistors located in the nondependent and dependent lungs. [150] This finding implies that when the pulmonary artery catheter tip is in the nondependent lung and the nondependent lung is ventilated, blood flow to the nondependent lung is undistorted or there is no interference with the function of the thermistor (or both).
When the pulmonary artery catheter is in the nondependent lung and the nondependent lung is ventilated
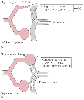 Figure 49-5
Conditions during thoracotomy in the lateral decubitus
position when pulmonary artery (PA) catheter data may be inaccurate. A,
During right thoracotomy with a PA catheter located in the collapsed right lung (one-lung
ventilation [1 LV]), cardiac output (CO) may be lower than when the right lung is
ventilated. The thermistor in the collapsed lung may be exposed to abnormal flow
patterns or vascular wall interference. B, When the
PA catheter is in the nondependent lung and the nondependent lung is exposed to continuous
positive airway pressure (CPAP) or positive end-expiratory pressure (PEEP), pulmonary
artery wedge pressure (Ppaw) may be inaccurate. Nondependent lung CPAP or PEEP may
cause zone 1 conditions in the nondependent lung. Ppaw is probably always reasonably
accurate when the PA catheter is in the dependent lung, even if the dependent lung
is exposed to PEEP. (From Benumof JL: Anesthesia for Thoracic Surgery.
Philadelphia, WB Saunders, 1987.)
Figure 49-5
Conditions during thoracotomy in the lateral decubitus
position when pulmonary artery (PA) catheter data may be inaccurate. A,
During right thoracotomy with a PA catheter located in the collapsed right lung (one-lung
ventilation [1 LV]), cardiac output (CO) may be lower than when the right lung is
ventilated. The thermistor in the collapsed lung may be exposed to abnormal flow
patterns or vascular wall interference. B, When the
PA catheter is in the nondependent lung and the nondependent lung is exposed to continuous
positive airway pressure (CPAP) or positive end-expiratory pressure (PEEP), pulmonary
artery wedge pressure (Ppaw) may be inaccurate. Nondependent lung CPAP or PEEP may
cause zone 1 conditions in the nondependent lung. Ppaw is probably always reasonably
accurate when the PA catheter is in the dependent lung, even if the dependent lung
is exposed to PEEP. (From Benumof JL: Anesthesia for Thoracic Surgery.
Philadelphia, WB Saunders, 1987.)
In summary, the LDP is important with regard to pulmonary artery catheter monitoring in three situations. First, when the nondependent lung is collapsed and the catheter is in the nondependent lung, the measured cardiac output and mixed venous blood PO2 (Pv̄O2 ) may be decreased in comparison to more normal conditions or the "real" value. Second, when the nondependent lung is ventilated with PEEP and the catheter is in the nondependent lung, Ppaw may not equal Pla. Third, when the catheter is in the dependent lung, Ppaw will be a faithful index of Pla, even if PEEP is used.
Finally, after pneumonectomy, inflation of the balloon of the pulmonary artery catheter to obtain Ppaw can result in considerable occlusion of the remaining cross-sectional area of the pulmonary circulation. This occlusion acutely decreases preload on the left ventricle and increases right ventricular afterload, thereby resulting in reduced cardiac output and reduced Pla. Although Ppaw under these circumstances still accurately reflects Pla, both these values have been artificially lowered by the blocked pulmonary circulation; hence, they result in a falsely low Ppaw reading.[152] This falsely low value for left ventricular filling pressure is misleading and may result in fluid management that contributes to the development of pulmonary edema and to the excessively high mortality reported in postpneumonectomy patients. Advancing the catheter carefully without inflating the balloon and wedging it into a smaller peripheral vessel can minimize the reduction in cross-sectional area of the pulmonary vasculature. Thus, a more accurate value for Ppaw can be obtained that reflects the true Pla.
|
|
|
|
|
|
|
|
|
|
|
|
|