|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thoracic surgical patients are at high risk for the development of postoperative pulmonary complications. In most of the medical literature, "postoperative complications" refers to the development of atelectasis or pneumonia, or both.[76] The incidence of pneumonia usually
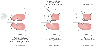 Figure 49-3
Thoracic surgery can impair postoperative lung function
because of preoperative, intraoperative, and postoperative factors (see the text
for details). (From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia,
WB Saunders, 1987.)
Figure 49-3
Thoracic surgery can impair postoperative lung function
because of preoperative, intraoperative, and postoperative factors (see the text
for details). (From Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia,
WB Saunders, 1987.)
Thoracic surgery promotes postoperative pulmonary complications for three main reasons; these reasons originate in the preoperative, intraoperative, and postoperative periods ( Fig. 49-3 ). First, the incidence of postoperative respiratory complications after any surgery is positively correlated with the degree of preoperative respiratory dysfunction, and most thoracic surgical patients come to surgery with some degree of preoperative lung dysfunction. When compared with nonsmokers, smokers have a 6-fold increase in the incidence of pulmonary complications after major operative procedures,[79] [80] and when compared with normal healthy patients, those with chronic lung disease have a 20-fold increased incidence of postoperative pulmonary complications.[81] Preoperative pulmonary function testing will identify patients at high risk because of poor preoperative lung function. The impact of this first factor (the presence of preoperative respiratory dysfunction) can be significantly reduced by preoperative prophylactic respiratory preparation measures (see later).[82] [83] [84] [85] [86]
The second reason why thoracic surgery promotes postoperative pulmonary complications is that the performance of surgery can impair lung function in any patient. During actual surgery, nondependent lung function may be impaired by resection of functional lung and by trauma to the remaining nondependent lung (as a result of various nondependent lung manipulations), and dependent lung function may be impaired as a result of the development of atelectasis and edema. This second factor can be minimized by appropriate intraoperative management (such as one-lung ventilation, PEEP, or continuous positive airway pressure [CPAP]—see later).
The third reason for increased pulmonary complications in these patients is that thoracotomy and upper abdominal incisions are most painful and cause patients to resist deep breathing and coughing in the postoperative period, which leads to retained secretions, atelectasis, and pneumonia.[82] [87] [88] [89] [90] [91] [92] [93] [94] It is painful for these patients to deep-breathe and to stretch either the chest or the abdominal wall (i.e., the incision); consequently, they fail to cough (which requires a deep breath), and they retain secretions (which promotes the development of atelectasis and infection). The third factor can be minimized by appropriate postoperative pain management (e.g., thoracic epidural analgesia) (see Chapter 72 ).
The preceding data indicate that patients undergoing thoracic surgery are particularly susceptible to postoperative respiratory complications[79] [80] [81] [82] [83] [87] [88] [89] [90] [91] [92] [93] [94] and that prophylactic measures do decrease such complications.[82] [83] [84] [85] [86] [93] [94] Consequently, preoperative evaluation should be followed by preoperative preparation efforts (see Chapter 25 ) directed toward optimally managing any preexisting pulmonary disease.[95] In general, a full preoperative respiratory preparation regimen involves a five-pronged attack on airway disease. The five elements of the preoperative regimen are stopping smoking, dilating the airways, loosening and removing secretions, and taking measures to increase motivation and education and facilitate postoperative care ( Table 49-7 and Fig. 49-4 ).
The five treatment modalities are instituted and proceed in parallel fashion. Before discussing each element separately, it is important to point out that the desirable results of these maneuvers should be, for the purpose of
| 1. Stop smoking, avoid industrial pollutants (if able to) |
| 2. Dilate airways |
| a. β2 -Agonists |
| b. Ipratropium bromide—especially if severe COPD |
| c. Inhaled steroids (systemic steroids—when bronchospasm is severe) |
| d. Cromolyn sodium—must institute before bronchospasm |
| 3. Loosen secretions |
| a. Airway hydration (humidifier/nebulizer) |
| b. Systemic hydration |
| c. Mucolytic and expectorant drugs |
| 4. Remove secretions |
| a. Postural drainage |
| b. Coughing |
| c. Chest physiotherapy (percussion and vibration) |
| 5. Adjunct medication |
| a. Antibiotics—if purulent sputum/bronchitis |
| b. Antacids, H2 blockers, or PPIs—if symptomatic reflux. |
| 6. Increased education, motivation, and facilitation of postoperative care |
| a. Psychological preparation |
| b. Preoperative pulmonary care training |
| 1. Incentive spirometry |
| 2. Secretion removal maneuvers |
| c. Preoperative exercise |
| d. Weight loss/gain |
| e. Stabilize other medical problems |
| COPD, chronic obstructive pulmonary disease; PPI, proton pump inhibitor. |
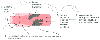 Figure 49-4
A full, aggressive, preoperative respiratory preparation
regimen consists of a five-pronged attack: (1) require the patient to stop smoking,
(2) dilate the airways, (3) loosen secretions, (4) remove secretions, and (5) increase
patient participation. Using these five maneuvers in the numbered sequence allows
them to complement one another in improving removal of secretions. IS, incentive
spirometer; PT, physical therapy. (From Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-4
A full, aggressive, preoperative respiratory preparation
regimen consists of a five-pronged attack: (1) require the patient to stop smoking,
(2) dilate the airways, (3) loosen secretions, (4) remove secretions, and (5) increase
patient participation. Using these five maneuvers in the numbered sequence allows
them to complement one another in improving removal of secretions. IS, incentive
spirometer; PT, physical therapy. (From Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
An improvement in mucociliary transport and small-airway function and a decrease in airway secretions and reactivity occur over a period of several weeks after cessation of smoking.[97] [98] Consequently, preoperative cessation of smoking for more than 4 to 8 weeks is associated with a decrease in the incidence of postoperative respiratory complications.[97] [98] Stopping smoking for only 24 hours will not decrease the amount of secretions (at least 1 to 2 weeks is required),[98] [99] airway irritability, and the incidence of postoperative respiratory complications. However, a number of important benefits accrue in the first 1 to 2 days of abstinence.[97] [98] [99] Cessation of smoking for as short a time as 12 to 48 hours has been shown to decrease carboxyhemoglobin levels significantly (increasing hemoglobin available for oxygen transport), shift the oxyhemoglobin dissociation curve to the right (increasing the availability of oxygen to the tissues),[100] and reduce nicotine-induced tachycardia[98] [99] ; in addition, cessation of smoking for a few days may greatly improve ciliary function. [98] [99] All these acute effects may confer a critical benefit to marginal patients.[84] Table 49-8 summarizes the benefits of smoking cessation for various durations before surgery.
For a few select patients, the risks of stopping smoking for 1 or 2 days may outweigh the benefits. These risks include excessive anxiety,[101] development of a hypersecretory and
| Time Course | Beneficial Effects |
|---|---|
| 12–24 hr | Decreased CO and nicotine levels |
| 48–72 hr | COHb levels normalized, ciliary function improves |
| 1–2 wk | Decreased sputum production |
| 4–6 wk | PFTs improve |
| 6–8 wk | Immune function and metabolism normalizes |
| 8–12 wk | Decreased overall postoperative morbidity and mortality |
| CO, carbon monoxide; COHb, carboxyhemoglobin; PFTs, pulmonary function tests. | |
The next preparatory step should be to dilate the airways (see Chapter 16 ). β2 -Agonists such as albuterol are administered to patients who have a demonstrable reversible bronchospastic airway component of their respiratory disease. Patients who have increased airway responsiveness and are therefore candidates for preoperative bronchodilation include smokers,[102] atopic individuals,[102] patients with airway symptoms of allergies,[102] patients with COPD,[103] [104] [105] [106] [107] [108] and asthmatics.[109] [110] The sympathomimetics (so-called first messengers) are believed to act on their target cells in the lungs by increasing the activity of adenyl cyclase, an enzyme in the cell membrane that catalyzes the formation of cyclic adenosine monophosphate (cAMP) from adenosine triphosphate (ATP). cAMP functions as a "second messenger" in the cell, where it acts on smooth muscle to decrease tension and motility. In addition, adrenergic compounds (epinephrine, ephedrine, isoproterenol) can increase ciliary activity, which may help in removing secretions.[111] [112]
cAMP is broken down by a cytoplasmic enzyme, phosphodiesterase, whose activity can be inhibited by methylxanthines such as theophylline and aminophylline. Thus, the methylxanthines also increase cAMP, but by a mechanism different from that of β2 -agonists. Because the methylxanthines and β2 -agonists act by different mechanisms, theophylline is often given to patients with bronchospasm who are already receiving β-adrenergics, and thus they work in synergy to increase intracellular concentrations of cAMP.[113] [114] In addition, aminophylline improves the contractility of the diaphragm and renders it less susceptible to fatigue.[115] Recently, however, there has been considerable concern about the short-term toxicity of methylxanthines; specifically, myocardial ischemia might occur as a result of the methylxanthines' effect on the heart, with resultant (and possibly fatal) ventricular arrhythmias. This effect may be exacerbated if β2 -agonists are used in addition. [116] [117] Therefore, appropriate caution should be exercised when combining inhaled β2 -agonists with methylxanthines.[118] Adrenocorticosteroids may also be given, by either the oral, parenteral, or aerosol routes, to the subgroup of patients with COPD who have especially reactive airways.[119] Steroids are not strictly categorized as bronchodilators, but they do have a bronchial-dilating effect, partly by decreasing mucosal edema and also by preventing the release of bronchoconstricting substances.
At present, there are two basic different approaches (plans) to the use of bronchodilators in asthma and COPD patients; the essential difference between the two approaches is whether the inhaled β2 -agonists or anticholinergics (or both) are considered front-line drugs with inhaled steroids being second-line drugs or vice versa (i.e., if inflammation is considered most important [120] [121] [122] ). Although the patient's subjective feeling of relief is an important end point, the effect of bronchodilator drug treatment should be quantified by pulmonary function tests.
The next step in preparing patients for thoracic surgery should be to thin and loosen thick adherent secretions. The most important method of thinning and loosening secretions is by hydration. When tracheal mucus transport velocity is quantitatively measured by radioactive tracer methods, it can clearly be shown that dehydration decreases and rehydration increases velocity.[123] The most common method of hydrating secretions is by the use of a humidifier or ultrasonic nebulizer to produce a heated, sterile water aerosol that is delivered by a close-fitting mask for 20 minutes to a patient breathing spontaneously with large tidal volumes. Concurrently, continuous systemic hydration must be ensured by adequate oral or intravenous intake.
Pulmonary infection, if present, is treated according to the results of culture and sensitivity tests; broad-spectrum antibiotics such as ampicillin or a cephalosporin frequently have the required specificity and potency. If the antibiotic treatment clears the infection to any extent, it may also decrease the tenacity, viscosity, and volume of secretions.
The next preoperative respiratory preparation step is the actual removal of secretions, which is accomplished by a combination of postural drainage (several different positions may be required), coughing, and chest percussion and vibration (common methods include tapping with cupped hands and the use of electric vibrators) for 15 to 20 minutes several times a day.[101] [124] [125]
Chest physiotherapy moves peripheral bronchial secretions to more central airways for expectoration by coughing.[126] The reason that coughing alone cannot clear peripheral airways is that an effective cough must attain a high enough airflow rate so that it shears secretions away from the airway wall. In patients with chronic lung disease, flow rates are low (especially peripherally), and shearing of secretions by cough may well be limited to the trachea and perhaps just the first two airway generations.[127] Obviously, with either chest physiotherapy
Chest physical therapy is relatively contraindicated in patients with lung abscesses, metastases to the ribs, a history of significant hemoptysis, and an inability to tolerate the postural drainage positions. It is generally agreed that intermittent positive-pressure breathing regimens do not have sufficient efficacy to warrant the excessive cost of routine use.[128] [129]
The forced expiration technique (FET) is increasingly being regarded as more effective in removing secretions than a cough is.[130] FET consists of a forced expiration starting from midlung volume (50% of inspiratory reserve lung volume) to a low lung volume, usually residual volume, followed by a period of relaxation and diaphragmatic breathing. This forceful expiration maneuver differs from a cough in that it is performed without closure of the glottis and without the accompanying compressive phase that characterizes a cough. Because transpulmonary pressure is less during FET than during a cough, airway compression is less and proximal and distal clearance of bronchial secretions is better than with a conventional cough. [130] FET is now commonly used as an alternative to cough during chest physiotherapy.[130]
Since Kennedy first reported the association between pulmonary symptoms and gastroesophageal reflux disease (GERD) almost 4 decades ago,[131] numerous papers have reported improvement of asthma symptoms with antireflux medication [132] and surgery.[133] Likewise, GERD has been increasingly appreciated as a risk factor for perioperative aspiration. These patients are presumed to chronically aspirate greater quantities of gastric contents during sleep than normal patients do. Many of these patients have reactive airway symptoms as a result of irritation of their airways. However, some investigators believe that the asthma-like symptoms are due to a vagally mediated esophagobronchial reflex.[134] Antireflux therapy alleviates the asthma symptoms but may not quantitatively improve pulmonary function test results.[135] Nonetheless, one recent study has demonstrated that antireflux surgery improves GERD and asthma symptoms, decreases asthma medication use, and improves pulmonary function in 90%, 79%, 88%, and 27%, respectively.[136]
Patients with GERD should receive preoperative non-particulate antacids (Bicitra) and gastropropulsive medications such as metoclopramide preoperatively, and cricoid pressure should be applied during induction of anesthesia. However, caution should be observed when administering H2 receptor blockers (famotidine, ranitidine) because of the possibility of provoking bronchospasm. Histamine mediates bronchoconstriction through the H1 receptor, whereas the H2 receptor mediates bronchial dilation. Indiscriminant use of H2 blockade could provoke unopposed bronchospasm through the H1 receptor. Thus, in patients with emphysema or reactive airways and coincident GERD (i.e., H2 blockers are considered important), H1 blockade (e.g., diphenhydramine [Benadryl]), ipratropium (Atrovent), and a β-agonist should be administered concurrently.
The last step of preoperative preparation consists of general measures designed to increase motivation and education and facilitate postoperative respiratory care. These measures include stabilization of all other medical conditions, psychological preparation, improvement of nutrition, exercise, weight loss in the obese, use of oxygen (when appropriate), antibiotics, instruction about realistic expectations of postoperative pain, and education regarding postoperative respiratory care procedures (including incentive spirometry, chest physiotherapy, postural drainage, and ambulation and exercise programs).
Lung expansion maneuvers such as deep-breathing exercises and the use of incentive spirometry are critical for limiting postoperative morbidity related to atelectasis and pneumonia. Furthermore, preoperative education on these lung expansion maneuvers has been shown to reduce post-thoracotomy pulmonary complications to a greater degree than when initial instruction is delayed until after surgery. The specific type of lung-expanding maneuver is not important; the critical factor is that it occur and patients be trained in the process preoperatively.[137]
Approximately 25% of patients undergoing thoracic surgery and pulmonary resection experience postoperative atrial dysrhythmias, of which atrial flutter/fibrillation is the most common.[138] The etiologic mechanisms of this complication are only partially understood. Resection of pulmonary tissue reduces the available pulmonary vascular bed for perfusion and can cause postoperative RV and right atrial enlargement. Thus, it is not surprising that the incidence of postoperative arrhythmias (as a result of atrial stretching) increases progressively with age and the amount of lung resected. In addition, the incidence of atrial arrhythmias is higher after left than after right pneumonectomy, possibly because of the greater degree of manipulation of the atrium with left lung surgery. However, age 60 years or older is the most consistent independent preoperative risk factor associated with postoperative atrial fibrillation/flutter.[138]
Several drugs have been administered in the perioperative period as prophylaxis against postoperative atrial fibrillation/flutter, including digoxin, calcium channel blockers (e.g., diltiazem), β-blockers, and amiodarone. Prophylactic diltiazem appears to be one of the best currently used options from a cost-benefit perspective.[139]
Indeed, a recent meta-analysis of 11 relevant randomized controlled trials revealed that overall, calcium channel blockers reduce major cardiac morbid events, with most benefits being attributable to diltiazem.[140]
Low-dose amiodarone (200 mg by mouth every 8 hours) has also been shown to be efficacious in reducing the incidence of atrial fibrillation after pulmonary resection.[141] With prolonged use, amiodarone is known to have numerous side effects, including pulmonary and endocrinologic effects. Additionally, acute lung injury has developed in a small number of patients undergoing thoracic operations after the administration of amiodarone.[142] Accordingly, further confirmation will be required before amiodarone
|
|
|
|
|
|
|
|
|
|
|
|
|