|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preoperative assessment of patients undergoing pulmonary resection must address three issues: first, is the tissue type amenable to surgery; second, is the spread confined enough that surgery will be curable; and third, is the patient fit for the planned operation? The cardiothoracic surgeon and pulmonologist usually decipher the answers to the first two questions (tissue type and staging) on their own. However, these issues have specific implications for the anesthesiologist as well. Accordingly, tissue typing and staging are briefly reviewed, and then the vast majority of this section covers evaluation of patient fitness for surgery (also see Chapter 25 ).
Both benign and malignant processes can be manifested as a lung mass, and only some of these lesions can be cured surgically. Benign pulmonary masses include hemangiomas, carcinoid tumors, sequestrations, nonmycobacterial granulomatous disease (e.g., Wegener's), and infectious processes (including bacterial pneumonia, tuberculosis, fungal mycetoma, and hydatid cyst). Viral and mycoplasma pneumonias are typically manifested as diffuse patchy alveolar infiltrates on chest radiography and are not treated by resection. Several of the aforementioned benign infiltrative processes do have specific anesthetic considerations (detailed later in the chapter).
The vast majority of pulmonary resections are performed for removal of malignant tissue. Accordingly, preoperative evaluation considerations should focus on issues relevant to the care of patients with lung cancer. Currently, lung cancer is the second most common type of malignancy encountered in the United States (15% of all cancer) and accounts for the greatest number of deaths in both men and women (28% of all cancer deaths).[3]
Bronchogenic carcinoma is a term commonly used to describe most lung cancers, but this term is imprecise because not all arise from the bronchi. Peripheral adenocarcinomas typically arise from bronchioli. Lung cancer is most logically divided into two major categories: small cell (a.k.a. oat cell, SCLC), which accounts for 20% to 25% of all primary lung cancer, and non-small cell (NSCLC), which accounts for the remaining 75% of cases.
The prognosis for SCLC is usually very poor, with an average survival of 3 months after diagnosis in untreated patients. Therapy for SCLC rarely involves surgery because these patients usually have disseminated disease—metastasis to bone (35%), liver (25%), central nervous system (CNS), lymph nodes, subcutaneous tissue, or pleura (10%)—at initial evaluation.[4] However, in some early-stage patients (T1-T2 N0 M0), surgical treatment followed by chemotherapy has been successful.[5] The only prospective, randomized phase III trial of SCLC found similar survival rates in patients receiving induction chemotherapy and radiotherapy, followed by surgery or observation. [6] Therefore, surgical resection cannot currently be recommended as standard treatment. Rather, surgery is reserved for a small group of select patients with early-stage disease, such as those who are found to have SCLC at thoracotomy (see Table 49-1 ). Regardless of the diagnostic setting, whenever lung biopsy reveals SCLC, these patients should be referred for chemotherapy within 1 week and have chemotherapy started within 2 weeks of diagnosis.[7]
Endocrinologic abnormalities and neurologic paraneoplastic syndromes are extremely common with SCLC. Forty percent of patients with SCLC manifest the syndrome of inappropriate antidiuretic hormone (SIADH) production. The enhanced secretion of atrial natriuretic hormone in these patients may further exacerbate this tendency toward hyponatremia, intravascular hypovolemia, and hypotension (particularly during induction of general anesthesia). Additionally, patients with SCLC may express the Eaton-Lambert myasthenic syndrome as a result of cross-reactivity between tumor-associated antigens and calcium-gated ion channels. These patients are at increased risk for prolonged neuromuscular blockade.[8]
NSCLCs are divided into six main types: (1) epidermoid or squamous cell (≅45%), (2) adenocarcinoma (≅20%), (3) large cell anaplastic (≅10%), (4) bronchial carcinoid (<5%), (5) alveolar cell carcinoma (<3%), and (6) sarcoma (<1%). Although the prognosis is variable, depending on the type of NSCLC and stage at diagnosis (see Table 49-1 ), they all respond to therapy in a similar way. Surgery is the only curative treatment of NSCLC and should be considered in all patients. However, less than 20% of NSCLC patients are initially seen at a stage that is amenable to surgical excision.[9] Furthermore, of patients with NSCLC who undergo surgery, less than 50% survive 5 years.[10]
Staging is the quantitative determination of the extent of tumor spread. The international TNM classification is broadly used for lung cancer. The TNM classification was first agreed on in 1986, revised in 1992, and revised again in 1997.[11] The TNM classification requires determination of tumor size (T), extent of nodal involvement (N), and the presence or absence of metastasis (M), as summarized in Table 49-2 .
Briefly, a T1 tumor is less than 3 cm in greatest dimension and is completely surrounded by lung or visceral pleura with no bronchoscopic evidence of invasion in any main bronchus. T2 indicates that the tumor is larger than 3 cm in diameter, invades the visceral pleura or main bronchi (but more than 2 cm from the carina), or is associated with obstructive atelectasis or pneumonia. Tumors that invade the parietal pleura, extend closer to the carina than 2 cm, or are associated with collapse of an entire lung are labeled T3. T4 tumors are those associated with a malignant pleural effusion or invasion of generally unresectable structures such as the heart, great vessels, trachea, vertebral body, carina, or esophagus. N0 indicates no nodal involvement; N1, ipsilateral peribronchial or hilar nodal involvement within the visceral pleura; N2, involvement of the ipsilateral mediastinal or subcranial
| Histologic Type of Lung Cancer | % of All Lung CA * | Is Surgical Resection Indicated | Comments (Including Smoking Association, and Prognosis) |
|---|---|---|---|
| Squamous cell (a.k.a. epidermoid) | 45% † | Yes—provided that stage < T3 N2 M0. Tends to metastasize locally and later than other bronchogenic carcinomas | Growth rate is more rapid than other NSCLCs. Associated with smoking. Prognosis is fair, good if caught early |
| Small cell lung carcinoma (a.k.a. oat cell) | 20–25% | Rarely—In early stage (T1-T2 N0 M0) in combination with postoperative chemotherapy, 80% respond to chemotherapy, but most relapse and die within 2 years. Radiation therapy is used for local control | Usually disseminated at diagnosis. SIADH and other endocrine or paraneoplastic syndromes common. Cigarette smoking accounts for >90% of cases. Prognosis is very poor |
| Adenocarcinoma (most common: bronchial origin; uncommon: bronchioloalveolar) | 15–25% † | Yes—provided that stage < T3 N2 M0 | Tend to be peripheral, slower growing, sometimes associated with areas of scarring, less closely associated with smoking. Prognosis is usually good |
| Large cell anaplastic | 10% | Yes—provided that stage < T3 N2 M0. May represent SCCA or adenocarcinomas too undifferentiated to identify cell type | Rapid growth rate and undifferentiated cell type. Is associated with smoking. Prognosis is poor |
| Bronchial carcinoid (a.k.a. bronchial adenoma) | <5% | Yes—provided that stage < T3 N2 M0. Slowly growing, but locally invasive | Usually age <40 years, occasionally metastasize. Prognosis is good |
| Alveolar cell carcinoma (bronchiolar) | <3% | Yes—provided that stage < T3 N2 M0. Behaves more like a benign expanding lesion because it rarely invades. | Native septal wall architecture preserved. Occurs in patients of all ages. Prognosis is usually good |
| Sarcoma | <1% | Yes—provided that stage < T3 N2 M0 | Arise from the connective tissue framework of lung. Prognosis varies |
| TNM relates to the international non-small cell lung carcinoma staging. T = primary size of tumor; N = nodal involvement; M = distant metastasis. Refer to text for details; also see Table 49-2 . The prognosis stated may be lower for advanced-stage disease. | |||
| NSCLC, non-small cell lung cancer; SCCA, squamous cell carcinoma; SIADH, syndrome of inappropriate antidiuretic hormone production. | |||
When each TNM value is summarized for a patient, a stage grouping can be assigned that correlates with prognosis and dictates operability. The stage groupings are also summarized in Table 49-2 . Surgery is usually indicated for stages I to IIIa but not for stages IIIb and IV. However, controversy exists regarding the proper staging of satellite lesions in the ipsilateral lobe of the primary lesion. In the 1992 criteria, such lesions would be a highgrade T designation; however, according to the 1997 revision, they are categorized as metastatic disease. Okada and colleagues have recently reported data indicating that ipsilateral satellite lesions should be reclassified according to the 1992 standards because the lesion would then be categorized as operable and thus improve survival.[12]
Despite significant advances, the diagnosis of lung cancer continues to portend a dismal overall prognosis. Of 100 patients with lung cancer, 65 will be nonoperable at initial evaluation. Of the 35 who receive operations, only 20 will have resectable disease. Of those 20 patients, 8 will be alive in 5 years and only 4 alive at 10 years.
The history (see Chapter 25 and Chapter 27 ) often raises the first suspicion of lung cancer. The average patient with cancer of the lung is in the sixth or seventh decade of life, has a history of heavy cigarette smoking and recent weight loss, and resides in an urban area. However, a small percentage of lung carcinomas occur in nonsmokers (<10%) (many of these can be traced to a history of passive or involuntary smoking),[13] and lung carcinoma has a higher incidence of occurrence in workers in some chemical industries (such as asbestos, arsenic, chromates, and nickel) than in the general population. Uranium miners have a greatly increased risk for the development of lung carcinoma, especially if they smoke. All age groups are affected, but the disease is rare in persons younger than 30 years. Five percent of patients are asymptomatic, and the tumor is discovered in this group only by routine roentgenographic
| Category | Class | Criteria Required for Classification |
|---|---|---|
| Primary size of tumor (T) | T0 | No evidence of primary tumor by cytology, bronchoscopy, or radiographic evaluation |
|
|
T1 | Tumor is 3 cm or less in greatest dimension, but completely surrounded by tissue |
|
|
T2 | Tumor > 3 cm in diameter, invades the visceral pleura, or has postobstructive atelectasis or pneumonia, but it does not extend within 2 cm of carina |
|
|
T3 | Tumor invades the parietal pleura, is associated with total lung collapse, and extends within 2 cm of (but does not involve) the carina |
|
|
T4 | Tumor associated with a malignant pleural effusion or invades generally unresectable structures (heart, great vessels, trachea or carina, vertebral bodies, esophagus) |
| Extent of nodal involvement (N) | N0 | No nodal involvement |
|
|
N1 | Involvement within the visceral pleura |
|
|
N2 | Involvement of nodes on the ipsilateral mediastinum |
|
|
N3 | Contralateral mediastinal, hilar, supraclavicular, or interscalene nodes |
| Evidence of metastasis (M) | M0 | No evidence of distant metastasis |
|
|
M1 | Distant metastasis present |
| Stage groupings † | Stage I | T<3 N0 M0 → Surgery indicated |
|
|
Stage II | T<3 N<2 M0 → Surgery indicated |
|
|
Stage IIIa | T<4 N<3 M0 → Surgery indicated |
|
|
Stage IIIb | T0-4 N0-3 M0 → No surgical benefit |
|
|
Stage IV | T1-4 N0-3 M1 → No surgical benefit |
| Adapted from Wilson WC: Anesthesia for thoracic surgery. In Kirby RR, Gravenstein N, Lobato EB, Gravenstein JS (eds): Clinical Anesthesia Practice, 2nd ed. Philadelphia, WB Saunders, 2002. | ||
Bronchopulmonary symptoms arising from involvement of the lung (cough, sputum, chest pain, dyspnea, wheeze) result from bronchial irritation, ulceration, obstruction, infection distal to the obstruction, or a combination of these processes. In a large series of patients with carcinoma of the lung, 75% had cough as one of the major symptoms, and this symptom was severe in 40% of patients. However, a cough is so common among cigarette smokers that many of them regard a morning cough as "normal." The most common stimulus to cough is the formation of sputum in the respiratory tract (see later), and the cough process is an essential element in keeping the tract clear.
A normal adult produces more than 100 mL of mucus from the respiratory tract each day. When excess mucus is formed, it may accumulate, stimulate the mucous membrane, and be coughed up as sputum. Sputum in patients with bronchogenic carcinoma may be formed in response to a physical, chemical, or infective insult to the mucous membrane of the airways. Mucoid sputum is clear or white. Black sputum most commonly results from the carbonaceous detritus of cigarette or atmospheric smoke. Purulent sputum typically results from bacterial infections but may result from viral or atypical organisms. Infections are more frequent after airway impingement (from malignant or nonmalignant causes). Failure to clear a recent change in the quality and quantity of sputum within a few days of initiating antibiotic therapy should raise suspicion of a neoplasm. Blood-stained sputum can vary from small streaks to gross hemoptysis (see later) and always warrants investigation for carcinoma. Hemoptysis, generally manifested as episodic blood streaking of the sputum, is present in 57% of patients with bronchogenic carcinoma and is often the first symptom.
Chest pain is present in 40% of patients evaluated for a new carcinoma. It is usually a mild, constant, dull ache on the side with the tumor. Another important form of chest pain with lung carcinoma is pleuritic pain. This pain is due to direct tumor extension to the pleura, is characteristically worse on breathing and coughing, and can usually be accurately localized by the patient. Mediastinal tumors can cause pain that is generally aching and retrosternal but poorly localized.
Dyspnea is a common complaint in patients with both chronic lung disease and lung carcinoma (30%). In chronic diseases, it is common to find that the patient begins to complain of dyspnea only after the respiratory reserve is quite severely impaired, whereas in patients with both acute infections and rapidly progressive lung carcinoma, dyspnea occurs more abruptly and with less objective functional impairment.
Wheezing is described by 10% of lung cancer patients and is frequently localized to one side. It is due to airway obstruction, and if the obstruction is located in the trachea or main bronchi, severe dyspnea and stridor may develop.
Other symptoms of chest disease result from growth of the tumor beyond the confines of the lung. These symptoms are due to involvement of the pleura (effusion), chest wall (pain), esophagus (dysphasia), superior vena cava (superior vena cava syndrome), pericardium (pericarditis), brachial plexus (arm pain), right or left recurrent laryngeal nerve (hoarseness), or bilateral recurrent laryngeal nerves (severe stridor). Additionally, apical lung cancer in the superior sulcus (known as Pancoast's tumor) can invade the cervical sympathetic plexus (causing pain in the distribution of the ulnar nerve), the subclavian vein (causing superior vena cava syndrome), or the cervical sympathetic nerves (causing Horner's syndrome—ipsilateral ptosis, miosis, exophthalmos, and anhidrosis). Approximately 15% of patients with carcinoma of the lung have these types of extrapulmonary intrathoracic symptoms.
Symptoms resulting from metastatic spread of tumor outside the thorax account for a small percentage of the initial or major complaints of patients with carcinoma of the lung. These extrathoracic metastatic symptoms can be referable, in order of general decreasing frequency, to the brain, skeleton, liver, adrenals, gastrointestinal tract, kidneys, and pancreas. The history with regard to these other organs is extremely important because any positive history referable to these organs requires specific organ workup for metastatic disease (for staging, see the section "Pulmonary Function Testing"), and detection of such metastases precludes curative surgery.
Extrathoracic nonmetastatic symptoms are usually due to a paraneoplastic syndrome caused by secretion of endocrine or endocrine-like substances by the tumor. Endocrinologic and paraneoplastic syndromes are occasionally the initial complaint in lung cancer patients. Whereas tumors secreting adrenocorticotropic hormone (Cushing's syndrome) and antidiuretic hormone (hyponatremia and SIADH) are more common with SCLC (oat cell), secretion of parathormone (and consequent hypercalcemia) is more common with squamous cell carcinoma.[14] [15] Bronchial carcinoids can cause carcinoid syndrome, although this syndrome is more common with gastrointestinal carcinoids that have metastasized to the liver. Endocrine-like manifestations include Cushing's syndrome, SIADH, carcinoid syndrome, hypercalcemia, ectopic gonadotropin secretion, and hypoglycemia. Neuromuscular manifestations consist of carcinomatous myopathies (Eaton-Lambert syndrome) and various myopathies related to brain dysfunction. Additional systemic manifestations of bronchial carcinomas include myopathies, neuropathies, other skeletal findings (e.g., clubbing, pulmonary hypertrophic osteoarthropathy), and vascular, hematologic (thrombophlebitis), and dermatologic (e.g., scleroderma, acanthosis nigricans) manifestations.[16]
Weight loss, anemia, weakness, anorexia, lethargy, and malaise occur in a large number of patients. Vague febrile respiratory (cold-like) syndromes may be present in 22% of these patients. In 10% to 15% of patients, these symptoms are responsible for the initial visit to the physician.
The basic tools of inspection, palpation, auscultation, and percussion only allow the physician to assess, in a gross way, the overall severity of chronic lung disease and the involvement of major consolidation, atelectasis, or pleural effusion and any obvious extrathoracic complications of thoracic carcinoma. It is now mandatory to use much more sensitive and specific radiographic (and other) means of determining resectability. Detection of pulmonary edema, airway compromise, and purulent secretions, however, is particularly important in that each requires further documentation, is usually reversible with treatment, and has important anesthetic management implications (discussed in the section "Preoperative Preparation").
Some of the routine laboratory tests (see Chapter 25 ) that are performed on all preoperative patients are especially relevant to the evaluation of a patient with a lung or bronchial mass. The complete blood count may indicate polycythemia, which may reflect chronically decreased arterial hemoglobin saturation; leukocytosis may be indicative of active pulmonary infection. Gram stain of sputum provides a qualitative index of infection, and cultures and sensitivity studies direct specific antibiotic therapy. Sputum cytology is useful in diagnosing neoplasms. Liver and bone enzymes, blood urea nitrogen and creatinine, and urinalysis can help establish the diagnosis of metastatic lung cancer. Computed tomography (CT) of the chest is the most sensitive and specific method for diagnosing both the presence and extent of lung cancer, and low-dose CT is now the best screening tool, whereas high-resolution CT is the staging tool of choice.[17] However, the chest radiograph is still the most common laboratory test performed that results in the diagnosis, and it is used for day-to-day evaluation of chest disease.
By the time that a tumor of the lung is first evident on a chest radiograph, it has probably completed three fourths of its natural history,[18] and this first radiographic abnormality frequently antedates the first symptoms or signs of the disease by 7 or more months.[19] By the time that bronchial carcinoma becomes symptomatic, the chest radiograph is abnormal in 98% of all patients, and the abnormality is most suggestive of tumor in over four fifths of these patients.
The radiographic findings of carcinoma of the lung ( Fig. 49-1 ) may be the result of the presence of the tumor within the lung[20] ( Table 49-3 ) (70% are centrally located), changes in the pulmonary parenchyma distal to a bronchus obstructed by the tumor (atelectasis, infection,
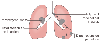 Figure 49-1
In patients with lung carcinoma, the chest radiographic
findings result from the presence of the tumor within the lung (parenchymal mass),
changes in the pulmonary parenchyma distal to a bronchus obstructed by the tumor
(atelectasis and infection), and spread of the tumor to extrapulmonary intrathoracic
sites (hilar and mediastinal masses and other direct extension pathology). (From
Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-1
In patients with lung carcinoma, the chest radiographic
findings result from the presence of the tumor within the lung (parenchymal mass),
changes in the pulmonary parenchyma distal to a bronchus obstructed by the tumor
(atelectasis and infection), and spread of the tumor to extrapulmonary intrathoracic
sites (hilar and mediastinal masses and other direct extension pathology). (From
Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
The usual radiographic manifestations of lung carcinoma frequently
include the hilar and extrapulmonary intrathoracic manifestations in addition to
the pulmonary parenchymal manifestations. In a review of the chest radiographs of
600 patients with carcinoma of the lung,[21]
the
average lung cancer mass at initial radiologic examination was 3 to 4 cm in diameter.
A large parenchymal mass was present in 22% of patients and a smaller mass in 20%;
multiple masses were found in only 1%. Obstructive pneumonitis, collapse, or consolidation
was
| More Likely Malignant |
| 1. Opacity larger than 3 cm in diameter |
| 2. Spiculated margins |
| 3. Noncalcified |
| 4. Increasing size (doubling time, 30–490 days) |
| More Likely Benign |
| 1. Stable size for 2 years † or doubling time less than 30 days (probably infectious) or more than 490 days (probably benign) |
| 2. Benign pattern of calcification |
| 3. Well-circumscribed margins |
| 4. Small (<2 cm) size |
| 5. Nearby satellite lesions |
| 6. Cavitated with thin walls or with air-fluid level |
| Indeterminate or Noncontributory Factors |
| 1. Age of lesion is unknown (no previous radiographs) |
| 2. Noncalcified or eccentric calcification |
| 3. Size 2–3 cm, with smooth margins |
| From Batra P, Brown K, Aberle DR, et al: Imaging techniques in the evaluation of pulmonary neoplasms. Chest 101:239, 1992. |
Several radiographic findings can have specific anesthetic implications.
These lesions consist of tracheal deviation or obstruction (difficulty with intubation
or ventilation); a mediastinal mass (difficulty with ventilation, superior vena cava
syndrome, compression of the pulmonary artery); pleural effusions (decreased vital
capacity and functional residual capacity [FRC]); cardiac enlargement (susceptibility
to anesthetics that depress the heart); bullous cyst (hazard of rupture, compression
of the adjacent lung); air-fluid levels (abscess with the hazard of spread of infection);
and parenchymal reticulation, consolidation, atelectasis, or edema (increased ventilation-perfusion
[V̇/![]() ] inequality and transpulmonary shunt). Additionally, up to 10% of
patients with chronic diffuse infiltrative lung disease may have normal chest radiographs.
[22]
] inequality and transpulmonary shunt). Additionally, up to 10% of
patients with chronic diffuse infiltrative lung disease may have normal chest radiographs.
[22]
The bronchoscopic examination is critical for staging, planning operative therapy, and deciding on the method of lung separation. Deviations from normal anatomy will have clear implications for preoperative planning, and thus the anesthesiologist must be appraised of the preoperative bronchoscopic findings.
Occasionally, for cost-saving reasons, certain patients with discrete lesions may have their bronchoscopic examination deferred to the operating room. The downside of deferring fiberoptic bronchoscopic examination is that an intrabronchial lesion may be found that requires biopsy, thereby potentially obviating surgical resection. When fiberoptic bronchoscopy is performed at this time, patients are subjected to significant additional risk and discomfort (placement of an arterial line, thoracic epidural, induction of general anesthesia). As mentioned previously, preoperative knowledge of any abnormal anatomy is critical for planning lung separation techniques. For these reasons, we believe that preliminary fiberoptic bronchoscopy should be performed by pulmonologists in advance of surgery.
Preoperative pulmonary evaluation (see Chapter 26 ) of patients with cancer of the lung should resolve questions concerning both resectability and operability. The question of resectability requires TNM staging of the disease and is based on clinical examination, radiographic (including CT) studies (T staging), bronchoscopic and mediastinoscopic examination (N staging), and evaluation and scanning of individual organs (M staging). Operability addresses the question of how much pulmonary tissue can be safely removed without rendering the patient a pulmonary cripple (the remaining lung may be diseased by a long history of smoking), and this question is usually answered by pulmonary function testing.
It is generally agreed that when pneumonectomy is being considered, pulmonary function testing should proceed in three phases ( Table 49-4 ).[23] [24] The first phase evaluates total lung function and consists of analysis of room-air arterial blood gases, as well as simple spirometry
| Testing Phase | PFT * | Increased Operative Risk Result |
|---|---|---|
| Whole-lung tests | Arterial blood gases | Hypercapnia on room air |
|
|
Spirometry | FEV1 < 50% of FVC |
|
|
|
FEV1 < 2 L |
|
|
|
MBC < 50% predicted |
|
|
Lung volume | RV/TLC > 50% |
| Single-lung tests | Right-left (individual-lung) split-function tests | Predicted postoperative FEV1 < 0.85 L or >70% blood flow to diseased lung |
| Mimic postoperative condition | Temporary unilateral balloon occlusion of right or left main stem bronchus or right or left pulmonary artery (must provide supplemental O2 ) | Mean pulmonary artery pressure >40 mm Hg, severe breathlessness, PaCO2 >60 mm Hg, or PaO2 <45 mm Hg |
| FEV1 , forced expired volume in first second; FVC, forced vital capacity; MBC, maximum breathing capacity; RV, residual volume; TLC, total lung capacity. | ||
| Pulmonary Function Test | Units and Designation (Preop Value vs Postop Prediction) | Normal | Pneumonectomy | Lobectomy | Segmental Resection |
|---|---|---|---|---|---|
| FEV1 | Liters (measured preop) | >4.0 | >2.1–1.7 | >1.2–1.0 | >0.6–0.9 |
|
|
% (measured preop) | >80% FVC | >50% FVC | >40% FVC | >40% FVC |
|
|
Liters (predicted postop) | N/A | >0.9–0.8 | >1 | >0.6–0.9 |
| FEV25–75% | Liters (measured preop) | >2 | >1.6 | 0.6–1.6 | >0.6 |
| FVC | Liters | >5.0 | >2.0 | — | — |
| MVV | Liters/min (measured for 1 min preop) | 100 | >50 | >40 | >25 |
|
|
% predicted (measured preop) | 100% | >50% | >40% | 25% |
| DLCO | % predicted (measured preop) | 100 | >60% | — | — |
|
|
% (predicted postop) | NA | >40% | — | — |
| Exercise testing | Stair climbing (measured preop) | >10 flights | >5 flights | >3 flights | >2 flights |
|
|
|
2.8 | >1 | >1 | >1 |
|
|
VO2 max (L/min) | None | <3% | <5% | <5% |
|
|
Oxy-Hb saturation drop with exercise |
|
|
|
|
| PaO2 | mm Hg (whole lung measured preop) | >90 | >80 | >70 | >60 |
| PaCO2 | mm Hg (whole lung measured preop) | 40 | <45 | <50 | <55 |
| FEV1 , volume of gas exhaled in the first second; FVC, forced vital capacity; FEV25–75% , mean expiratory flow rate in the middle half of the FVC maneuver; MVV, maximum voluntary ventilation; DLCO, diffusing capacity; VO2 max, maximum oxygen consumption. Volume and VO2 max values are based on a 70-kg adult. | |||||
| Criteria are based on data from refs [31] [32] [33] [34] . | |||||
If the second-level criterion of acceptable predicted postoperative FEV1 cannot be met and surgery is still contemplated or desired, the postoperative condition of the patient can be simulated (the third phase of testing) by functionally resecting the vascular bed of the lung to be excised by temporary balloon occlusion of the major pulmonary artery on that side, with and without exercise.[30] Under these conditions, the distensibility (compliance) of the remaining pulmonary vascular bed is tested, and an increase in mean pulmonary artery pressure to greater than 40 mm Hg, an increase in PaCO2 above 60 mm Hg, or a decrease in PaO2 to less than 45 mm Hg (or any combination of these three criteria) indicates an inability to tolerate removal of this amount of lung.
Ventilatory function after pneumonectomy (or after any resection) can also be simulated preoperatively by passing, with the aid of a fiberoptic bronchoscope, a balloon occlusion catheter that can occlude either lung (or any lobe) and then performing spirometry of the remaining lung tissue (after careful withdrawal of the bronchoscope). Supplemental oxygen must be administered during bronchial blockade because the blocked segment would still be perfused and all this perfusion would be right-to-left shunt flow, which would create a risk of hypoxemia.
This pulmonary function testing cascade is logical because it starts out with simple, relatively inexpensive, noninvasive tests and increases the degree of difficulty, expense, and invasiveness only as indicated.
Although less restrictive pulmonary function test criteria for the performance of pulmonary resections less radical than pneumonectomy have been published (see Table 49-5 ), [31] [32] [33] [34] [35] [36] [37] there are several reasons why, at least in some patients, it may be prudent to think of a lobectomy (and lesser procedures) as a functional pneumonectomy.[38] First, during the immediate postoperative period, the function of the lung tissue remaining on the operative side may be significantly impaired by atelectasis and perhaps infection; consequently, these patients may experience significant transient postoperative functional impairment.[39] Patients who are most likely to have a stormy postoperative course with minor resections are those who have had intraoperative exposure problems that required severe and prolonged lung manipulation. Intraoperative exposure problems are most likely to occur when the lung being operated on is large and moving (large tidal volume with positive-pressure ventilation). Second, at the time of thoracotomy, more accurate staging of the disease is possible, and it may become apparent that it is necessary to perform a pneumonectomy. Third, the function of the lung on the nonoperated side may be impaired preoperatively[39] and may acutely deteriorate intraoperatively as a result of spillage of blood or pus, or both, from the operated to nonoperated lung or by an inability of the nonoperated lung to tolerate a prolonged period of dependency and compression in the LDP. Finally, postlobectomy function studies have shown that although ventilation and perfusion of the lung remaining on the operated side become significantly greater during the long-term interval (3 to 51 months), the volume of the remaining lung gradually increases and becomes significantly greater than the increase in either ventilation or perfusion to this lung.[40] The compensatory hyperinflation represents dilation of the preexisting respiratory units without disruption or fragmentation of the elastic tissue as seen in pathologic emphysema; however, the pulmonary hyperinflation decreases compliance and therefore the ventilation per unit volume of the ipsilateral remaining pulmonary tissue. In addition, the hyperinflated lung stretches and thins out the capillaries in the alveolar walls, thereby decreasing the perfusion per unit volume of the ipsilateral remaining pulmonary tissue.
The vast majority of patients with pulmonary tumors have had a long history of smoking; consequently, they have varying degrees of chronic obstructive pulmonary disease (COPD). The cardiovascular response to the pathologic alveolar and airway changes in COPD consists of the development of pulmonary hypertension and increased pulmonary vascular resistance (PVR), followed by right ventricular (RV) hypertrophy and dilation.
Increased PVR has important implications for patients undergoing pulmonary resection. Whereas a normal pulmonary vasculature is distensible and capable of accommodating large increases in pulmonary blood flow (to approximately 2 to 2.5 times normal, as would occur through the remaining lung after pneumonectomy) with only minor increases in pulmonary artery pressure ( Fig. 49-2 ), the relatively rigid and restricted pulmonary vascular bed of patients with chronic lung disease cannot accommodate even small increases in pulmonary blood flow without concomitant increases in PVR.[41] The inability to tolerate increases in blood flow occurs over the entire range of physiologic cardiac output and may be an important contributing factor to the development of postpneumonectomy pulmonary edema when it occurs.[42]
The preoperative pulmonary function testing cascade as outlined in pulmonary function testing phases 1 and 2 in Table 49-4 (which in reality, is the extent to which the great majority of patients are studied preoperatively) does not allow the diagnosis of increased PVR and RV disease. Increased PVR may be noninvasively suspected preoperatively by the presence of auscultatory and radiographic signs of pulmonary hypertension and by electrocardiographic evidence of right atrial and ventricular hypertrophy ( Table 49-6 ). The development of a positive hepatojugular reflex, ascites, and peripheral edema indicates the onset of cor pulmonale. In COPD patients without waking hypoxemia, cor pulmonale can be detected twice as sensitively and frequently by echocardiography (criteria for detection are pulmonary hypertension and
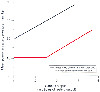 Figure 49-2
Mean pulmonary artery pressure (y
axis) does not increase until cardiac output (x axis)
has increased 2- to 2.5-fold when the pulmonary vascular bed is normal, whereas mean
pulmonary artery pressure increases linearly with increasing cardiac output when
the pulmonary vascular bed is restricted. (From Robin ED, Gaudio R: Cor
pulmonale. Dis Mon May:3–38, 1970.)
Figure 49-2
Mean pulmonary artery pressure (y
axis) does not increase until cardiac output (x axis)
has increased 2- to 2.5-fold when the pulmonary vascular bed is normal, whereas mean
pulmonary artery pressure increases linearly with increasing cardiac output when
the pulmonary vascular bed is restricted. (From Robin ED, Gaudio R: Cor
pulmonale. Dis Mon May:3–38, 1970.)
| Auscultatory Signs of ↑PAP and ↑PVR | Radiographic Signs of ↑PAP and ↑PVR | Electrocardiographic Signs of ↑RA and ↑RV | Additional Signs of CP |
|---|---|---|---|
| ↑Pulmonary component of second heart sound | Dilation of main pulmonary artery | ↑RV | All those of ↑PAP, ↑PVR, ↑RA, ↑RV |
|
|
|
Clockwise vector rotation |
|
|
|
|
Right axis deviation |
|
|
|
|
↑R and ↑ing S wave V2 –V6 |
|
|
|
|
Inverted T wave V1 –V6 |
|
| Loss of normally present split in second heart sound | Fullness of apical pulmonary vessels |
|
Pulmonary diastolic murmur |
| Presence of fourth heart sound | Counterclockwise cardiac rotation: globular shape on PA film (the RV comprises the left and right heart border, aortic knob) | ↑RA | Third heart sound |
|
|
|
↓ST segment V2 –V6 |
|
|
|
|
↑P wave II and III; diphasic P wave V1 |
|
| Appearance of high-pitched early systolic ejection click | Lateral film showing encroachment of retrosternal air space (RV dilation) |
|
Prominent right sternal border pulsation plus retraction over left side of chest → rocking motion synchronous with heartbeat. Chronic dependent edema, large tender liver, ascites, distended neck veins (large A waves) |
| ↑, increased; ↓, decreased; →, leads to; CP, cor pulmonale; PA, posteroanterior; PAP, pulmonary hypertension; PVR, increased pulmonary vascular resistance; RA, right atrial hypertrophy; RV, right ventricular hypertrophy. | |||
Measurements of PVR have been made directly by determining mean pulmonary artery and pulmonary artery wedge pressure at various levels of cardiac output produced by varying treadmill exercises. Thus, by using the patient's own cardiac output, pulmonary vascular compliance can be determined. PVR measurements made in this way have been good indicators of the risk associated with pneumonectomy. [44] [45] Operative risk was considered to be increased if PVR was greater than 190 dyne/sec/cm. However, if the risk, expense, and time to pass a pulmonary artery catheter have been accepted, it is logical to take one further step and measure pulmonary vascular pressure during temporary unilateral pulmonary artery balloon occlusion in states of rest and exercise (see pulmonary function testing, phase 3). This maneuver specifically tests the compliance of just the pulmonary vascular bed that will remain after pneumonectomy. Performing this procedure during exercise is the most realistic preoperative approximation of the pulmonary vascular and RV function to be expected in an ambulatory postpneumonectomy patient.[23] [30] Also, echocardiography is increasingly being used to estimate RV alterations and pulmonary hypertension (see earlier).[43]
In addition to the preoperative condition of the pulmonary vasculature, the intraoperative anesthetic and surgical experience can introduce numerous other causes of further acute increases in PVR, including episodes of
The possible independent causes that may contribute to left ventricular (LV) dysfunction in patients with lung disease consist of coronary artery or valvular disease,[55] [56] systemic hypertension,[55] [56] presence of carboxyhemoglobin,[57] systemic hypoxemia and acidosis,[58] marked alterations in intrathoracic pressure,[59] [60] and RV dysfunction.[55] [61] [62] [63] [64] In view of the usual age, the long and heavy smoking history, and the frequently sedentary lifestyle of patients undergoing thoracic surgery, it is not surprising that coronary artery disease is by far the most likely independent cause of LV dysfunction. Myocardial ischemia leading to infarction may occur throughout the perioperative period, although peaks of incidence occur during surgery and on the third day after surgery. The first peak is caused by intraoperative changes in hemodynamics and the second peak by episodes of hypoxia, uneven administration of pain medication, and withdrawal or alteration of drug therapy.[65]
Only two preoperative clinical predictors of perioperative cardiac morbidity (defined as the occurrence of myocardial infarction, unstable angina, congestive heart failure, serious dysrhythmia, or cardiac death during the intraoperative or in-hospital postoperative periods) have been definitively confirmed: recent (<6 months) myocardial infarction and current congestive heart failure.[66] The classic historical intraoperative predictors of morbidity—emergency surgery, prolonged (>3 hours) operations, and thoracic or upper abdominal surgery—also appear to be independent predictors of perioperative morbidity, whereas the choice of anesthetic is not. The dynamic intraoperative predictors of perioperative cardiac morbidity are intraoperative hypotension and tachycardia. Hypertension remains a controversial predictor.
If a history of angina is present or the electrocardiogram (ECG) is suggestive, further preoperative evaluation of coronary artery function is necessary (suggestive evidence includes Q waves [previous infarction], left bundle branch block, ST-segment elevation [transmural ischemia], ST-segment depression [subendocardial ischemia], T-wave inversions, and a positive U wave [left main coronary artery disease]). The first step should be noninvasive exercise testing. ECG and thallium scans (in that order) appear to be the best such exercise tests at this time. An exercise study provides information about the functional level of the patient. Unfortunately, the degree of exercise stress may be limited by low ventilatory reserve, as well as by low cardiac reserve. If the exercise ECG is normal, surgery should proceed; if the exercise ECG indicates ischemia, a thallium exercise test is indicated.[67] If the thallium exercise test is negative, the planned pulmonary resection should proceed; if the thallium exercise scan is positive for ischemia, coronary angiography should be performed.[68] However, if for any reason it is strongly suspected that the patient is indeed having significant angina, even though exercise testing is negative or equivocal, coronary angiography is indicated. Consideration should always be given to coronary angiography in a patient with proven previous myocardial infarction, especially if the patient currently has angina. Echocardiography is increasingly being used to estimate LV function.
If significant coronary artery disease is present, the patient needs coronary artery bypass grafting before or at the time of pulmonary resection. For lesser degrees of coronary artery disease, pulmonary resection for carcinoma of the lung should be performed after appropriate medical therapy for coronary insufficiency has been initiated. If the patient needs coronary artery bypass grafting and limited resection can encompass the cancer, both procedures can be performed under the same anesthetic, but the coronary artery bypass grafting should be done before pulmonary resection.[69] [70] After bypass, if the patient is stable, has good myocardial function, and is not bleeding, a pulmonary wedge resection can be performed. For patients who require coronary artery bypass grafting and have pulmonary lesions that require segmentectomy, lobectomy, or pneumonectomy, there is a good possibility that the prolonged nature of the pulmonary procedures will increase operative mortality (and therefore should not be done), although a small number of successful combined procedures have been reported.[70] [71] In one series of 21 patients, the pulmonary mass was discovered on the preoperative chest x-ray film (for cardiac surgery) and therefore before the occurrence of any symptoms and by definition constituted a fortuitously early diagnosis. Not surprisingly, resection at the time of cardiac surgery (17 wedge resections, 4 lobectomies) resulted in a 95% 5-year survival rate.[72]
In cases that require large resections in compromised patients, coronary artery bypass grafting should be done first, and pulmonary resection should be delayed until the patient has gained weight and muscle mass (usually 4 to 6 weeks). The risk associated with general anesthesia for a noncardiac operation in a patient with previous coronary artery bypass grafting is similar to that in patients without proven coronary artery disease.[73] [74] Although it is not possible to estimate the true effects of a delay in pulmonary resection in terms of tumor spread in a possibly immunocompromised patient (especially after general anesthesia),[75] it seems reasonable that in the latter group (those requiring bypass grafting and major pulmonary resection), the operative risk of combined procedures probably exceeds the risk of tumor spread.
|
|
|
|
|
|
|
|
|
|
|
|
|