Chapter 12
- Intravenous Drug Delivery Systems
- Peter S. A. Glass
- Steven L. Shafer
- J. G. Reves
Introduction
For anesthetic drugs to be effective, they must reach their site
of action. In 1628, William Harvey proved in Exercitatio Anatomica
de Motu Cordis et Sanguinis in Animalibus that venous blood was transported
to the arterial circulation and thus to the organs of the body by the heart. It
was thus recognized almost immediately that drugs injected into veins could be rapidly
carried to the entire body. Indeed, in 1657, Christopher Wren injected opium intravenously
by means of a quill and bladder ( Fig.
12-1A
) in dogs and humans and rendered them unconscious. Eight years later,
Sigismund Elsholtz gave an opioid solution for the purpose of rendering subjects
insensitive, but it was not until 1874 that Pierre-Cyprien Ore administered chloral
hydrate intravenously for a surgical procedure. This landmark occasion followed
unsuccessful attempts by the Russian surgeon Pirogoff to administer ether intravenously
in 1846, shortly after Crawford Long and William Morton had independently demonstrated
the efficacy of ether inhalation for surgery.[1]
Intravenous methods of anesthetic drug delivery have depended
on a steady improvement in technology. The quill and bladder used by Wren were not
significantly improved on until Alexander Wood used a needle and syringe to administer
intravenous medications in 1853. The hollow hypodermic needle was developed by Frances
Rynd, and a functional syringe, by Charles Pravaz.[1]
Contemporary needles, catheters, and syringes are descendants of these early devices.
The latest technologic development in intravenous anesthesia has been the introduction
of computerized pharmacokinetic model-driven continuous-infusion devices ( Fig.
12-1B
), first published by Helmut Schwilden[2]
in 1981. Schwilden demonstrated the ability to attain desired plasma levels of an
intravenous anesthetic drug by using a computer-controlled infusion pump driven by
the published pharmacokinetics of the drug. These efforts resulted in the release
in Europe of the first commercial target-controlled infusion (TCI) device, developed
by Zeneca, specifically for the administration of propofol. The March 1998 issue
of the journal Anaesthesia was devoted entirely to
a review of TCI, with a focus on the "Diprifusor" and the role of TCI devices in
clinical practice.
The ultimate development in anesthetic delivery systems will be
devices for closed-loop administration of intravenous drugs during anesthesia. Systems
have been developed for closed-loop administration of sodium nitroprusside[3]
[4]
[5]
[6]
[7]
and muscle relaxants[8]
[9]
[10]
[11]
[12]
[13]
[14]
(see Chapter 13
). The drug
effect is easily measured for vasoactive drugs and muscle relaxants, thus facilitating
closed-loop control. Until recently, the lack of an unequivocal measure of "anesthesic
depth" has hindered the development of closed-loop anesthetic delivery systems.
Clinical assessment of anesthetic depth requires integration of the patient's physiology,
the drug dosing history, the level of
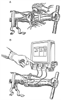 Figure 12-1
Intravenous drug delivery, past and future. A,
Depiction of the first intravenous injection of opium with a quill and bladder.
B, The future of intravenous drug delivery, in which
drugs are delivered with the aid of a small, sophisticated infusion pump that permits
dosing in terms of plasma drug concentration rather than amount.
Figure 12-1
Intravenous drug delivery, past and future. A,
Depiction of the first intravenous injection of opium with a quill and bladder.
B, The future of intravenous drug delivery, in which
drugs are delivered with the aid of a small, sophisticated infusion pump that permits
dosing in terms of plasma drug concentration rather than amount.
noxious stimulation, and measurement of the patient's response to stimulation (hemodynamics,
movement, electroencephalogram [EEG], tearing, and so on), as discussed in Chapter
31
. Increasing understanding of the physiology involved in providing anesthesia
and the development of monitoring devices for specific components of the anesthetic
state have resulted in increasing efforts to develop such closed-loop devices. Schwilden
and colleagues have developed closed-loop systems for the infusion of methohexital
[15]
[16]
and propofol
[17]
on the basis of the median EEG frequency.
Several
other investigators have now also developed closed-loop systems based on derivatives
of the EEG (e.g., auditory evoked potentials[18]
or the bispectral index[19]
developed by Aspect
Medical Systems[20]
).
The development of new techniques has coincided with the development
of new drugs. Rapid intravenous induction became popular with the introduction of
sodium thiopental in 1934. The pharmacokinetics of thiopental prevented it becoming
popular for the maintenance of anesthesia. During the past 50 years, numerous intravenous
hypnotics (methohexital, 1957; propanidid, 1957; althesin, 1971; etomidate, 1973;
propofol, 1977), anxiolytics (diazepam, 1966; midazolam, 1978), and analgesics (fentanyl,
1959; ketamine, 1966; sufentanil, 1979; alfentanil, 1980; remifentanil, 1996) have
been introduced. The general trend in the introduction of these newer drugs has
been shorter and shorter times for recovery from drug effect. The newer intravenous
anesthetics propofol, etomidate, alfentanil, sufentanil, and remifentanil have all
been shown to provide a rapid onset of anesthesia, a stable maintenance phase, and
rapid recovery.
Before a review of intravenous anesthesia delivery techniques
and devices, we need to review pharmacokinetic and pharmacodynamic principles to
understand how to administer intravenous drugs to their best advantage. Therefore,
we will develop these concepts and then show how intravenous anesthesia delivery
systems can be used to rationally dose the intravenous drugs used in clinical practice.
Further discussion of the principles of pharmacokinetics and pharmacodynamics can
be found in Chapter 3
.
 Figure 12-1
Intravenous drug delivery, past and future. A,
Depiction of the first intravenous injection of opium with a quill and bladder.
B, The future of intravenous drug delivery, in which
drugs are delivered with the aid of a small, sophisticated infusion pump that permits
dosing in terms of plasma drug concentration rather than amount.
Figure 12-1
Intravenous drug delivery, past and future. A,
Depiction of the first intravenous injection of opium with a quill and bladder.
B, The future of intravenous drug delivery, in which
drugs are delivered with the aid of a small, sophisticated infusion pump that permits
dosing in terms of plasma drug concentration rather than amount.