|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Having established that we can produce almost any desired concentration time course at the effect site, what concentration should we choose? The pharmacokinetics and pharmacodynamics of inhaled anesthetics are greatly simplified by the equilibrium established at the alveolar gas/blood interface, which permits measurement of the minimum alveolar concentration (MAC) associated with a 50% likelihood of movement in response to noxious stimulation.[35] Considerable effort has gone into developing a concept equivalent to MAC for intravenous anesthetic drugs.
The pharmacodynamics of intravenous anesthetics are reported in terms of C50 , the concentration that produces 50% of the maximum possible drug effect. There are many ways of thinking about C50 . It might be the drug concentration that prevents a response (e.g., movement, hypertension, and catecholamine release) to a particular stimulus (e.g., incision, intubation, and sternal spreading) in 50% of patients. In this case, each combination of stimulus and response may have a unique C50 . For example, Ausems and colleagues defined the C50 of alfentanil in the presence of 66% nitrous oxide for several noxious stimuli.[36] When C50 is defined as the drug concentration that produces a given response in 50% of patients, it is also the concentration associated with a 50% probability of response in a given patient. Note that defining C50 as the concentration that produces a given effect in 50% of individuals implicitly assumes that the effect can be achieved in all individuals. Some drugs exhibit a ceiling effect.
Several studies have been performed to establish appropriate concentrations for intravenous anesthetics. The C50 values of alfentanil, fentanyl, and remifentanil in the presence of 70% nitrous oxide have been defined for skin incision ( Table 12-2 ). C50 values for loss of consciousness and skin incision have also been defined for the hypnotics thiopental,[37] [38] propofol,[39] [40] [41] and midazolam (see Table 12-2 ).[42] Defining the C50 , like MAC, provides a measure of relative potency between the intravenous anesthetics.
Another interpretation of C50 is the concentration that produces 50% of the maximum possible physiologic response. For example, the C50 for an EEG response is the drug concentration that provides 50% of the maximum EEG effect. The C50 for EEG response has been measured for the opioids alfentanil,[43] fentanyl,[43] sufentanil,[44] remifentanil,[45] [46] [47] and trefentanil. [48] It has also been determined for thiopental, [37] [49] [50] etomidate,[51] propofol,[52] and benzodiazepines (see Table 12-2 ). [53]
Although C50
values for all combinations of stimuli
and measures have not been experimentally determined for each opioid, they can be
accurately estimated by scaling the values of C50
determined experimentally
for other opioids by their relative potency.[54]
For example, the C50
| C50 Drug | C50 for EEG Depression † | C50 for Incision or Painful Stimulus ‡ | C50 for Loss of Consciousness § | C50 for Spontaneous Ventilation ¶ | C50 for Isoflurane MAC Reduction | MEAC |
|---|---|---|---|---|---|---|
| Alfentanil (ng/mL) | 500–600 | 200–300 | — | 175–225 | 50 | 10–30 |
| Fentanyl (ng/mL) | 6–10 | 4–6 | — | 2–3 | 1.67 | 0.5–1 |
| Sufentanil (ng/mL) | 0.5–0.75 | (0.3–0.4) | — | (0.15–0.2) | 0.145 | 0.025–0.05 |
| Remifentanil (ng/mL) | 10–15 | 4–6 | — | 2–3 | 1.23 | 0.5–1 |
| Thiopental (µg/mL) | 15–20 | 35–40 | 8–16 | — | — | — |
| Midazolam (ng/mL) | 250–350 | — | 125–250 | — | — | — |
| EEG, electroencephalogram; MAC, minimum alveolar concentration; MEAC, minimum effective plasma concentration providing postoperative analgesia. | ||||||
To be entirely independent of dosing history, the C50 must be determined at steady state, which is rarely possible because most anesthetic drugs do not reach steady state during a continuous infusion until many hours have passed. However, if the drug achieves rapid equilibration between plasma and the effect site and the investigator waits long enough after starting the infusion, this choice can be reasonably satisfactory. For example, Ausems and colleagues[36] [55] used a continuous infusion of alfentanil, which equilibrates quickly, in their experiments. They also took their measurements after the effect-site concentration had equilibrated with the plasma concentration.
A second alternative to performing a true steady-state experiment is to use mathematical modeling to calculate the effect-site concentrations of drug at the time of the measurement, as proposed by Hull and colleagues[25] and Sheiner and coworkers.[26] The relationship between effect-site and plasma concentrations is represented graphically in Figure 12-5 and mathematically in Equation 6. Calculating effect-site concentrations is nothing more than attempting to determine the steady-state plasma concentrations that would produce the observed drug effect. Sometimes, when the C50 reflects effect-site concentrations, it is represented as Ce50 to distinguish it from values of C50 that are based on plasma
A third alternative to performing a steady-state experiment is to establish a pseudo-steady state by the use of computer-controlled drug delivery. This method has become the state-of-the-art means for determining the C50 for anesthetic drugs, and many of the C50 values referenced earlier were determined at pseudo-steady state by the use of computer-controlled drug delivery. Typically, a constant plasma concentration steady state must be maintained for four to five plasma effect-site equilibration half-lives (e.g., 10 to 15 minutes for fentanyl). Such a long delay is not necessarily required when computer-controlled drug delivery is used. For example, Glass and associates[56] used computer-controlled infusions to achieve initially higher plasma target concentrations to provide more rapid acquisition of the desired effect-site concentration. This process can be automated by having the computer actually target the concentration in the effect site rather than plasma and thereby rapidly establish plasma-effect-site equilibration.[30] [31]
Thus, there are several ways to establish C50 in terms of steady-state concentrations. C50 can be estimated through mathematical effect-site modeling or can be measured experimentally by the use of computer-controlled drug delivery to quickly establish a pseudo-steady state. Either way, when performing studies to define the concentration-effect relationship, equilibrium must exist or be modeled for between the biophase (the site of effect) and plasma or blood (where the concentration is actually measured).
When C50 is defined in terms of the concentration associated with a response in half of a population, that same C50 is the concentration associated with a 50% probability of response in a typical individual. However, individual patients are not typical individuals but instead will have their own C50 values. Expressed in clinical terms, different patients have different anesthetic requirements for the same stimulus. For example, the minimal effective analgesic concentration of fentanyl is 0.6 ng/mL but varies among patients from 0.2 to 2.0 ng/mL.[57] The minimal effective analgesic concentrations of alfentanil[58] [59] and sufentanil[60] similarly vary among patients by a factor of 5 to 10. This range encompasses both variability in the intensity of the stimulus and variability in the individual patient. However, this wide range reflects the clinical reality that must be accounted for when dosing regimens are designed. Because of this variability, intravenous anesthetics should be titrated to each patient's unique anesthetic requirement for a given stimulus.
A common residency experience for the authors, and perhaps for the reader as well, was an attempt to administer "pure" techniques: pure inhalational anesthesia (e.g., isoflurane/oxygen), pure nitrous/narcotic anesthesia (nitrous oxide/fentanyl/oxygen), pure ketamine anesthesia, and so on. We rapidly discovered that pure hypnotic techniques required huge doses to achieve anything close to a satisfactory anesthetic. The only benefit of struggling with pure techniques is the appreciation one gets for the essential role of drug interactions in the practice of anesthesia.
Drug interactions cause the C50 of one drug to shift in response to administration of a second drug. We have already referred to the ability of opioids to reduce MAC, a clinically useful drug interaction with a long history. In 1901, George Crile suggested that opioids should be administered with supplemental drugs for intravenous anesthesia. In 1959, DeCastro and Mundeleer introduced the term "neurolept anesthesia," which consists of a tranquilizer, opioid, and nitrous oxide. Today, the term coined by John Lundy, "balanced anesthesia," is used to describe the concurrent administration of several anesthetic drugs so that no single drug is given in a dosage sufficient to produce toxicity during or after surgery. Contemporary anesthesia consists of at least two components—analgesia and loss of consciousness. Combining an opioid with a volatile anesthetic or intravenous hypnotic provides both components. The interaction of hypnotics and opioids in producing the anesthetic state is complex but predictable.
Drug interactions are species dependent. Thiopental and opioids show relative antagonism to analgesic end points in rats,[61] [62] whereas their effects are synergistic in humans. [63] Just as C50 must be defined for very specific end points, drug interactions must also be clearly defined in terms of end points. For example, Kissin and coworkers observed that "the combination of a barbiturate and an opioid gives different outcomes for different endpoints of anesthesia."[62] Often, we differentiate between analgesic end points (i.e., analgesia to noxious stimulation) and hypnotic end points (e.g., sedation and loss of consciousness). In a study of morphine-midazolam interactions on sedation and loss of the righting reflex in rats, Kissin and colleagues[64] observed that "hypnotic" end points differ. Although sedation and loss of the righting reflex appear to be hypnotic end points, these authors found that they were associated with different degrees of synergy between morphine and analgesia, which led to the conclusion that "differences in the outcomes of midazolam-morphine interactions regarding sedation and hypnosis (loss of righting reflex) suggest that underlying mechanisms for these two effects are different. Therefore, they should not be regarded as only increasing depths of the same action."
The MAC of volatile anesthetics appears to be additive when several inhaled anesthetics are administered concurrently.[65] [66] The additivity of MAC suggests a uniform mechanism of action for the inhaled anesthetics, although the "unitary theory" of inhalational anesthesia has fallen into disfavor.[67] [68] The intravenous anesthetics encompass categories of drugs (hypnotics, opioids, psychotomimetics, anxiolytics, neuroleptics, local anesthetics, and so on) with different receptors and mechanisms of action. In the absence of a uniform mechanism of action, there is no reason to anticipate simple additivity, for example, that maintaining a 30% C50 of drug A plus a 40% C50 of drug B will produce the same effect as a 70% C50 of either drug A or drug B.
The assumption of balanced anesthesia is that drug combinations will be synergistic in anesthetic effect (however defined) but not in toxicity. Such synergism has
Vuyk and colleagues characterized the interaction between propofol and alfentanil for several end points: loss of response to intubation, loss of response to intraoperative stimulation, and emergence from anesthesia ( Fig. 12-8 ).[40] The most profound stimulation was tracheal intubation, and abolition of that stimulus required a propofol concentration of at least 2 µg/mL. Vuyk and associates also documented an interaction of propofol and alfentanil on emergence from anesthesia, a sedative end point.
Mean alveolar concentration is defined as the steady-state alveolar concentration of anesthetic gas that inhibits movement during incision in 50% of patients. It is an excellent measure of potency. MAC is also a useful guideline for clinical dosage, provided that the clinician remembers that it is the concentration in 50% of patients that inhibits a movement response and that it is defined in terms of an analgesic end point—loss of movement in
 Figure 12-7
The interaction of propofol and fentanyl in providing
loss of consciousness. The solid lines represent the concentrations of propofol
and fentanyl when administered together that are required to prevent a response to
a verbal command in 50% of patients at each decade of age.
Figure 12-7
The interaction of propofol and fentanyl in providing
loss of consciousness. The solid lines represent the concentrations of propofol
and fentanyl when administered together that are required to prevent a response to
a verbal command in 50% of patients at each decade of age.
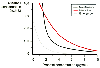 Figure 12-8
Interaction between alfentanil and propofol on three
different end points: response to intubation (red line),
maintenance of anesthesia (black line), and concentrations
associated with emergence from anesthesia (gray line).
The curve shows the concentrations associated with
a 50% probability of the respective end point. (Adapted from Vuyk J, Lim
T, Engbers FH, et al: The pharmacodynamic interaction of propofol and alfentanil
during lower abdominal surgery in women. Anesthesiology 83:8–22, 1995.)
Figure 12-8
Interaction between alfentanil and propofol on three
different end points: response to intubation (red line),
maintenance of anesthesia (black line), and concentrations
associated with emergence from anesthesia (gray line).
The curve shows the concentrations associated with
a 50% probability of the respective end point. (Adapted from Vuyk J, Lim
T, Engbers FH, et al: The pharmacodynamic interaction of propofol and alfentanil
during lower abdominal surgery in women. Anesthesiology 83:8–22, 1995.)
MAC reductions of isoflurane by fentanyl,[71] sufentanil,[72] alfentanil,[73] and remifentanil[74] have all been defined. Concentrations of these opioids that provide a 50% MAC reduction are listed in Table 12-2 . Probably the most commonly used combination of anesthetics is isoflurane and fentanyl. McEwan and coworkers[71] demonstrated that the MAC of isoflurane is reduced by 50% at a fentanyl concentration of 1.7 ng/mL ( Fig. 12-9 ), which corresponds to a fentanyl loading dose of 4 µg/kg followed by a 1.75-µg/kg/hr infusion. Because the minimum effective analgesic concentration of fentanyl is 0.6 ng/mL[57] and because clinically significant respiratory depression may occur with plasma fentanyl concentrations above 2 ng/mL,[75] it is fortunate indeed that the steepest reduction in MAC occurred within the useful analgesic range for fentanyl (i.e., 0.6 to 2 ng/mL). In other words, just maintaining fentanyl within an analgesic range reduces MAC by 50%. The study by McEwan and colleagues also demonstrated that beyond a fentanyl plasma concentration of 5 ng/mL, a plateau is seen with a maximal MAC reduction of approximately 80%. The maximal reduction in isoflurane was to a concentration of ±0.3%, that is, a value close to the awake MAC for isoflurane.[76]
Alfentanil,[73] sufentanil,[72] and remifentanil[74] produce similar reductions in isoflurane MAC, with an initial steep
 Figure 12-9
Interaction of isoflurane and fentanyl in preventing
a somatic response at skin incision (i.e., minimum alveolar concentration [MAC] reduction
of isoflurane). The solid line represents the concentrations
of isoflurane and fentanyl, when administered together, that are required to prevent
purposeful movement in 50% of patients at skin incision. The dashed
lines represent the 95% confidence interval of the MAC at each combination
of fentanyl and isoflurane. (From McEwan Al, Smith C, Dyar O, et al: Isoflurane
MAC reduction by fentanyl. Anesthesiology 78:864–869, 1993.)
Figure 12-9
Interaction of isoflurane and fentanyl in preventing
a somatic response at skin incision (i.e., minimum alveolar concentration [MAC] reduction
of isoflurane). The solid line represents the concentrations
of isoflurane and fentanyl, when administered together, that are required to prevent
purposeful movement in 50% of patients at skin incision. The dashed
lines represent the 95% confidence interval of the MAC at each combination
of fentanyl and isoflurane. (From McEwan Al, Smith C, Dyar O, et al: Isoflurane
MAC reduction by fentanyl. Anesthesiology 78:864–869, 1993.)
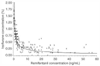 Figure 12-10
Interaction of isoflurane and remifentanil in preventing
a somatic response at skin incision (i.e., minimum alveolar concentration reduction
of isoflurane). The solid line represents the concentrations
of isoflurane and remifentanil, when administered together, that are required to
prevent purposeful movement in 50% of patients at skin incision. From Lang
E, Kapila A, Shlugman D, et al: Reduction of isoflurane minimal alveolar concentration
by remifentanil. Anesthesiology 85:721–728, 1996.)
Figure 12-10
Interaction of isoflurane and remifentanil in preventing
a somatic response at skin incision (i.e., minimum alveolar concentration reduction
of isoflurane). The solid line represents the concentrations
of isoflurane and remifentanil, when administered together, that are required to
prevent purposeful movement in 50% of patients at skin incision. From Lang
E, Kapila A, Shlugman D, et al: Reduction of isoflurane minimal alveolar concentration
by remifentanil. Anesthesiology 85:721–728, 1996.)
 Figure 12-11
Relationship between a response surface and a standard
isobologram. Conventional "isobolographic" analysis, whether for doses or concentrations,
describes only the concentrations of both drugs that yield a 50% drug effect and
thus fails to capture the entire response surface. (From Minto CF, Schnider
TW, Short TG, et al: Response surface model for anesthetic drug interactions. Anesthesiology
92:1603–1616, 2000.)
Figure 12-11
Relationship between a response surface and a standard
isobologram. Conventional "isobolographic" analysis, whether for doses or concentrations,
describes only the concentrations of both drugs that yield a 50% drug effect and
thus fails to capture the entire response surface. (From Minto CF, Schnider
TW, Short TG, et al: Response surface model for anesthetic drug interactions. Anesthesiology
92:1603–1616, 2000.)
We have examined the relationship between opioids and hypnotics on the C50 , the concentration of drug associated with 50% of the maximal effect. It is important to realize that the interaction between two drugs actually describes a surface where each drug has its own axis, and the third axis is the effect from any combination of the two drugs. Figure 12-11 shows the relationship between the three-dimensional response surface and the more commonly shown relationship in two dimensions between the C50 of two drugs. Minto and colleagues published "response surfaces" for combinations of midazolam-alfentanil, propofol-alfentanil, and midazolam-propofol
 Figure 12-12
Response surface for each of the paired interactions
between propofol and midazolam (A), alfentanil and
midazolam (B), and alfentanil and propofol (C)
on the probability of opening eyes to a verbal command. The isoboles for a 10%,
20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90% response are shown. (From Minto
CF, Schnider TW, Short TG, et al: Response surface model for anesthetic drug interactions.
Anesthesiology 92:1603–1616, 2000.)
Figure 12-12
Response surface for each of the paired interactions
between propofol and midazolam (A), alfentanil and
midazolam (B), and alfentanil and propofol (C)
on the probability of opening eyes to a verbal command. The isoboles for a 10%,
20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90% response are shown. (From Minto
CF, Schnider TW, Short TG, et al: Response surface model for anesthetic drug interactions.
Anesthesiology 92:1603–1616, 2000.)
There is still variability in sensitivity from patient to patient, as well as variability in the level of stimulation associated with different surgical procedures. Thus, each patient is an experiment, with the clinician learning the individual patient's C50 for the combination of drugs used during the administration of each anesthetic. Despite this variability, dosing guidelines play an important role in the practice of anesthesia. The concentration ranges of the intravenous anesthetics for anesthesia, sedation, and analgesia are given in Table 12-3 . These ranges provide starting estimates on which subsequent titration can be based. They also provide a means of estimating whether the patient is having a typical or an atypical response. If the patient's dosing requirements deviate greatly from established guidelines, it is reasonable to determine whether something else might be going on with either the patient or the drug delivery system.
|
|
|
|
|
|
|
|
|
|
|
|
|