|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CRAO decreases the blood supply to the entire retina; occlusion of a retinal arterial branch (BRAO) is a localized injury that affects only a portion of the retina. Mechanisms of perioperative retinal ischemia include increased ocular venous pressure (e.g., when venous drainage from the eye is impaired or IOP is increased) or a reduction in arterial supply (e.g., because of emboli or systemic hypotension), or both. Venous drainage can be impaired after radical neck surgery if the jugular vein or veins are ligated. External pressure, such as from an improperly used headrest, could increase IOP. Pressure within the orbit could also be increased internally after retrobulbar hemorrhage. A reduction in arterial blood supply to the retina sufficient to produce ischemia can result from cardiac surgery if emboli
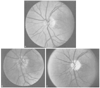 Figure 82-8
Funduscopic images showing the time course of changes
in anterior ischemic optic neuropathy (AION). A,
Normal fundus. B, Initial optic disk edema in AION.
C, Optic atrophy in the late stages. (From
Hayreh SS: Ischemic optic neuropathy. University of Iowa, Department of Ophthalmology
Internet Site. http://webeye.ophth.uiowa.edu/dept/aion/ion_fgD.jpg.)
Figure 82-8
Funduscopic images showing the time course of changes
in anterior ischemic optic neuropathy (AION). A,
Normal fundus. B, Initial optic disk edema in AION.
C, Optic atrophy in the late stages. (From
Hayreh SS: Ischemic optic neuropathy. University of Iowa, Department of Ophthalmology
Internet Site. http://webeye.ophth.uiowa.edu/dept/aion/ion_fgD.jpg.)
 Figure 82-9
The electroretinogram (ERG) in central retinal arterial
occlusion. The ERG is recorded under varying scotopic conditions. The ERG is shown
before, during, and after ischemia, up to 7 days later. (From Roth S, Li
B, Rosenbaum PS, et al: Preconditioning provides complete protection against retinal
ischemic injury in rats. Invest Ophthalmol Vis Sci 39:777, 1998).
Figure 82-9
The electroretinogram (ERG) in central retinal arterial
occlusion. The ERG is recorded under varying scotopic conditions. The ERG is shown
before, during, and after ischemia, up to 7 days later. (From Roth S, Li
B, Rosenbaum PS, et al: Preconditioning provides complete protection against retinal
ischemic injury in rats. Invest Ophthalmol Vis Sci 39:777, 1998).
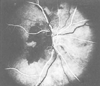 Figure 82-10
Fluorescein angiography in anterior ischemic optic neuropathy.
The dark areas correspond to absence of filling
of the choroid and optic disk with fluorescein dye. (From Hayreh SS: Ischemic
optic neuropathy. University of Iowa, Department of Ophthalmology Internet Site.
http://webeye.ophth.uiowa.edu/dept/aion/ion_fgF.jpg.)
Figure 82-10
Fluorescein angiography in anterior ischemic optic neuropathy.
The dark areas correspond to absence of filling
of the choroid and optic disk with fluorescein dye. (From Hayreh SS: Ischemic
optic neuropathy. University of Iowa, Department of Ophthalmology Internet Site.
http://webeye.ophth.uiowa.edu/dept/aion/ion_fgF.jpg.)
Case reports in the literature comprised 32 patients ( Table 82-2 ). In this group, 43% of the cases followed neck, nasal, or sinus surgery, and 25% occurred after spine surgery. Pressure from the headrest in the prone position or from the anesthesia mask was the suspected cause of the visual symptoms in about half the cases. As a group, these patients were healthy, with a mean age of 38 years and a low incidence of systemic disease. Some patients were thought to have a low nasal bridge that may have predisposed them to damage from external pressure on the eye. Hypotension and anemia were not generally reported in these cases, although in most reports, blood pressure and hemoglobin/hematocrit values were not provided. Some of the cases followed intra-arterial injections of corticosteroids or local anesthetics into branches of the external carotid artery, with possible retrograde embolization to the ocular blood supply. One patient had signs of external pressure. Three cases followed coronary artery bypass graft (CABG) surgery and had visible retinal emboli. Of the eight cases after spine surgery, mean blood pressures of 73, 70, and 50 mm Hg were reported in three patients. Symptoms generally occurred within the first postoperative day. Some of the patients with CRAO but none with BRAO had accompanying symptoms such as eye muscle paresis, periorbital edema, bruises, or proptosis. Except for one patient, no improvement was found after CRAO; after BRAO, some improvement was usually noted.
Retinal ischemia is a common mechanism of injury resulting in blindness or severe visual disability in vascular diseases such as retinal vascular occlusion, retinopathy of prematurity, and diabetes.[124] Most instances of profound visual loss in the United States are caused by diseases that ultimately result in retinal ischemia. Findings of increased extracellular glutamate concentrations during retinal ischemia[125] and attenuation of ischemic injury in vitro and in vivo by glutamate receptor antagonists [126] [127] [128] support a role for excitotoxicity in the retina. It is believed that an increased intracellular Ca2+ concentration as a result of enhanced glutamate release ultimately initiates mechanisms that result in cellular destruction.
In addition to ischemia-related chemical alterations, two distinct flow patterns follow a period of ischemia. In cats, retinal and choroidal blood flow increases dramatically (hyperemia) immediately after the end of ischemia.[45] Adenosine and nitric oxide are responsible for this hyperemia.[129] [130] [131] Hyperemia is of clinical relevance when reperfusion occurs after a period of ischemia; increased flow when vessels or the blood-retinal barrier is damaged might lead to macular edema. [132] Alternatively, hyperemia may represent a necessary physiologic adjustment to a previous profound disturbance in blood flow. Hypoperfusion is the other extreme in blood flow derangement. Delayed retinal hypoperfusion 1 to 4 hours after the end of a period of ischemia has been shown in adult rats,[133] and these results resemble the low-flow state found in other models after global cerebral ischemia.[134] [135] The mechanism of these changes in blood flow has not been clearly established; although depletion of vasodilators such as adenosine or nitric oxide may be responsible, a predominance of vasoconstrictors such as endothelins cannot be excluded.[63]
Altered gene expression may also contribute to pathogenesis. For example, increased activity of the tyrosine
|
|
AION | PION | CRAO/BRAO |
|---|---|---|---|
| No. of cases | 51 | 38 | 32 |
| Authors | Alpert 1987[67] | Alexandrakis 1999[93] | Bekar 1996[109] |
|
|
Brown 1994[68] | Asensio 2002[94] | Bradish 1986[110] |
|
|
Busch 1998[69] | Balm 1990[286] | Cheney 1987[111] |
|
|
Chisholm 1969[70] | Brown 1994[68] | Gillan 1953[112] |
|
|
Chun 1997[71] | Chisholm 1969[70] | Girotto 1998[113] |
|
|
Dilger 1998[72] | Chutkow 1973[95] | Givner 1950[114] |
|
|
Gotte 2000[73] | Dodd 1993[285] | Grossman 1985[287] |
|
|
Gupta 2002[74] | Dunker 2002[96] | Grossman 1993[115] |
|
|
Jaben 1983[75] | Johnson 1987[55] | Gutman 1971[116] |
|
|
Janicki 2001[76] | Katz 1994[77] | Hollenhorst 1954[117] |
|
|
Katz 1994[77] | Lee 1995[97] | Locastro 1991[118] |
|
|
Kirkali 1990[78] | Lee 2001[98] | Lund 1994[119] |
|
|
Larkin 1987[79] | Lo 2002[99] | Plate 1981[120] |
|
|
Minagar 2000[80] | Marks 1990[100] | Savino 1990[82] |
|
|
Moster 1998[81] | Mazzia 1962[101] | Torti 1964[121] |
|
|
Savino 1990[82] | Milner 1960[102] | Whiteman 1980[122] |
|
|
Sha'aban 2000[83] | Remigio 2000[103] | Wolfe 1992[123] |
|
|
Shahian 1989[84] | Rizzo 1987[104] |
|
|
|
Shaked 1998[85] | Roth 1997[105] |
|
|
|
Shapira 1996[86] | Savino 1990[82] |
|
|
|
Sharma 1993[87] | Schobel 1995[106] |
|
|
|
Stevens 1997[88] | Stevens 1997[88] |
|
|
|
Strome 1997[89] | Tsai 1997[107] |
|
|
|
Sweeney 1982[90] | Warner 2001[2] |
|
|
|
Williams 1999[91] | Wessels 1987[108] |
|
|
|
Wilson 1991[92] |
|
|
In humans, the retinal blood supply is derived from the retinal and choroidal vessels.[10] [139] Occlusion of both the retinal and choroidal circulations would be extremely unusual in humans. Therefore, after retinal vascular occlusion, some oxygen may still be supplied by diffusion from the outer retinal layers by way of the choroid. Oxygen and a supply of glucose in the vitreous may account for the relatively lengthy survival time of the retina after ischemia shown in primate studies.[140] Moreover, cells of the inner retina such as ganglion cells are more susceptible to ischemic damage than the photoreceptor cells of the outer retina are, even in animals when both the retinal and choroidal blood supplies are occluded.[132] [141] [142] The implication is that there may be a relative selectivity to ischemic damage in the retina. However, in humans, this finding may also be due in part to the choroidal circulation supplying primarily the photoreceptors in the outer retina. Whatever the mechanism, the retina differs from the cerebral cortex in that longer periods of ischemia can be tolerated, a characteristic that renders the retina amenable to delayed treatment after ischemia. By implication, a patient with retinal ischemia in the postoperative period should be aggressively treated in an attempt to salvage vision.
Various etiologies of CRAO have been proposed by Wray[14] and by Rettinger and colleagues.[143] The mechanisms include (1) emboli, (2) atheromatous disease in vessels with superimposed thrombosis or hemorrhage, (3) inflammation (e.g., giant cell arteritis), (4) vasospasm, and (5) arterial occlusion as a result of high IOP or low retinal perfusion pressure.
Some patients with altered facial anatomy may be predisposed to damage caused by external pressure from anesthesia masks or headrests. Osteogenesis imperfecta is associated with blue sclerae. Fibrous coats of the eye are thin and immature because of a deficiency of collagen fibers, persistent reticulin fibers, and increased mucopolysaccharide ground substance. Sclerae and corneas are unusually thin, and exophthalmos is common as a result of bony facial abnormalities. These factors render
Even in patients with normal anatomy, improper positioning of the head may cause compression of the ocular and periorbital contents and, consequently, occlusion of retinal blood flow.[109] [115] [117] In older reports of verified external pressure on the eye during mask anesthesia and hypovolemic shock (abdominal surgery and ureterostomy), CRAO was found in combination with corneal abrasion. On induction of anesthesia, the patient complained of pressure from the anesthesia mask on the eye, and edema of the eyelids and conjunctiva was found postoperatively.[112] [114] The risk of direct compression of the eye is of particular concern during surgery performed in the prone position.[115] [123] In older reports, CRAO was found in patients positioned prone with the use of a horseshoe headrest. Proper use of more modern head-positioning devices such as square or circular foam headrests with cutouts for the eyes should prevent compression of the eyes, but cases continue to be reported despite the use of rectangular headrests.[144] Adding to the risk during procedures performed in the prone position is the use of deliberate hypotension and a steep head-down position because both factors could further reduce ocular perfusion pressure. However, whether the presence of the latter two factors alone could themselves lead to retinal arterial or venous occlusion has never been documented.
Retinal venous hemorrhage has been described in an otherwise healthy young woman who immediately on awakening from anesthesia complained of seeing red. By the end of surgery the patient had frequent premature ventricular contractions that disappeared with hyperventilation. Funduscopy revealed a venous retinal hemorrhage involving the macula. The patient recovered fully, and the tentative diagnosis was Valsalva hemorrhagic retinopathy, a rare entity in which vomiting, "stormy" emergence from anesthesia, or blowing up air mattresses is thought to cause retinal venous hemorrhage. The postulated mechanism in this case was a combination of retinal vasodilation, increased intracranial pressure as a result of hypoxemia and hypercapnia, and obstruction of retinal venous return by the Valsalva maneuver.[145] However, this mechanism does not result in permanent visual loss.
Neck surgery and nasal/sinus surgery account for the highest proportion of reported cases of CRAO postoperatively (see Table 82-2 ). During nasal or sinus surgery, direct damage to the vascular system of the eye may cause thrombosis or spasm in arteries, or the CRA may be compressed by retrobulbar hematoma after iatrogenic fracture of the lamina papyracea.[146] Indirect damage to the CRAO from intra-arterial injections of 1% lidocaine with epinephrine[82] [111] or lidocaine mixed with vasopressin[143] has also been described; the mechanism of action is speculated to be arterial spasm or embolism. [82] Embolization to cerebral cortical vessels may also occur inasmuch as one case was associated with transient confusion and left-sided weakness.[111]
In two reports, CRAO after spinal surgery and radical neck dissection was accompanied by periorbital edema or chemosis and ocular muscle dysfunction, thus suggesting impaired venous drainage and compression injury of the extraocular muscles. [121] [123] External pressure resulting from the prone position or bilateral surgical removal of the external jugular veins, respectively, may have been the cause. The presence of such facial signs and symptoms may be useful in differentiating RAO from ION.
BRAO usually leads to permanent ischemic retinal damage with partial visual field loss. Symptoms may not be noticed by the patient immediately when only peripheral visual field loss or a small scotoma is present. BRAO is primarily the result of emboli of various origin, but vasospasm has been reported in a few cases. Most case reports describe embolization of material from intravascular injections and circulating embolic material from the surgical field or bypass equipment in cardiac surgery.
Microemboli to the retina during cardiopulmonary bypass have been shown by retinal fluorescein angiography (also see Chapter 50 ). The occurrence and extent of perfusion defects were related to oxygenator type; when a bubble oxygenator was used, all patients had perfusion defects indicative of microemboli, whereas when a membrane oxygenator was used, retinal perfusion defects were found in only half the patients. Unfortunately, the study did not present neurologic outcome.[65] In CABG surgery patients, multiple calcific emboli in branches of the CRA are not unusual,[14] and these emboli may produce visual field deficits of varying size and location. A preliminary study in seven patients during heart valve surgery showed retinal emboli, some temporary, in all patients. None of these emboli resulted in visual defects in surviving patients, but one patient had findings of concomitant cortical blindness.[147]
Case reports have described sudden irreversible blindness with BRAO in patients after the injection of various drugs into the head and neck region. Several authors reported nearly instantaneous loss of vision when injecting steroids in the nasal mucosa.[122] [148] [149] [150] In approximately half the reported cases, crystalline emboli could be seen at funduscopy, and in one incident, vasospasm was apparently present. This injury appears to be preventable. Mabry[150] summarized 100,000 injections without neurologic or ophthalmologic injury and pointed out that knowledge of the anastomosis between terminal branches of the OA and the external carotid system should prompt a refined technique for reducing the risk of intra-arterial injections and embolization to the ophthalmic system. To produce retrograde flow into the OA branches, the injection needle must be located intra-arterially and the perfusion pressure must be overcome during injection. Consequently, topical nasal vasoconstrictors should be applied to reduce the size of the vascular bed, and a small (25 gauge) needle on a low-volume syringe should be used to minimize injection pressure.
Two case reports describe visual loss after injections of combinations of corticosteroids, penicillin, and lidocaine into the tonsillar fossae and steroid, lidocaine, and epinephrine into the pterygopalatine fissure to relieve
Local infiltration anesthesia with lidocaine or bupivacaine in combination with epinephrine (1:100,000 or 1:200,000) for nasal septal surgery can cause partial or total visual field defects postoperatively that are attributable to BRAO.[82] [120] No emboli were visible at funduscopy in these cases. It was speculated that the cause of BRAO was vasospasm induced by accidental intra-arterial retrograde injection of epinephrine or the combination of lidocaine and epinephrine into branches of the external carotid artery. Another possible contributing factor might have been epinephrine-induced platelet aggregation with retrograde embolization.[82] Although both mechanisms are speculative, this latter possibility is supported by a case report of verified platelet hypersensitivity to epinephrine. The patient used a sympathomimetic spray (oxymetazoline) chronically as a nasal decongestant. After an acute incident of decreased visual acuity, funduscopy revealed a fibrin platelet embolus in a retinal artery branch.[152]
In a canine model, intracarotid injections of lidocaine, epinephrine, and corticosteroid were administered. Injection of 2 mL of lidocaine produced no visible funduscopic change. Injection of 2 mL of 1:100,000 epinephrine alone or with lidocaine produced transient vasospasm. An injection of partly dissolved steroid (with particles) and 1:100,000 epinephrine or epinephrine/lidocaine caused permanent retinal ischemic damage. The authors concluded that injection of particulate matter was necessary to produce permanent damage.[153] Platelet-induced hypercoagulability appears to play a role as well.[152] The human ocular circulation differs from that of the canine: in humans, a single CRA supplies the retina; in dogs, the retinal circulation is derived from multiple branches of the posterior ciliary circulation. Accordingly, the canine retinal circulation may be somewhat more protected from the deleterious effects of arterial embolism and occlusion than that of humans.
One unusual case report described BRAO after elective termination of pregnancy in the first trimester. The next day the patient noted decreased vision in one eye. Retinal findings were suggestive of BRAO. The patient had a patent foramen ovale, and a diagnosis of amnionic fluid emboli to a cilioretinal artery was suspected.[154]
Perioperative RAO resulted in permanent loss of vision in most of the reported cases.[14] The diagnosis will not ordinarily be sought in the absence of patient symptoms. Slow awakening from anesthesia will generally delay the recognition of symptoms. In studies in monkeys, Hayreh and Weingeist[140] found that the critical time limit of irreversible retinal damage is approximately 100 minutes. CRAO lasting 105 minutes produced irreversible damage, whereas recovery was seen if ischemic time was limited to 97 minutes. Shorter periods of retinal ischemia have been associated with varying degrees of recovery in animal studies.[141] [142] The recovery pattern after BRAO would be expected to be similar, but there is more likely to be some remaining visual function.
The currently available methods of treatment are not satisfactory. Some initial treatment consisting of ocular massage to lower IOP (contraindicated if glaucoma cannot be ruled out) and thereby dislodge an embolus to more peripheral arterial branches could potentially be instituted by the anesthesiologist.[14] Intravenous acetazolamide should be administered to increase retinal blood flow, [153] and the patient may inhale 5% CO2 in oxygen to enhance dilation and increase oxygen delivery from retinal and choroidal vessels.[10] Further treatment should be prescribed by the ophthalmologist and may include thrombolysis, although such treatment may be relatively contraindicated after certain surgical procedures. A preliminary clinical study showed that fibrinolysis within 6 to 8 hours through a catheter in the OA was associated with improved visual outcome.[156] Localized application of hypothermia to the affected eye is a simple technique that has been shown to decrease injury in animal studies after ischemia,[157] and it is probably reasonable to institute in humans because of its minimal risk.
To prevent retinal vascular occlusion from external pressure, the anesthesiologist must avoid compression of the globe. Pressure on the eye from anesthetic masks should be avoidable. If surgery is near the face, the surgeon's arm must not be allowed to rest on the patient's eye. In patients positioned prone for surgery, a padded headrest should be used with the eyes properly placed in the opening of the headrest. Intermittent examination of the eyes is advisable while the patient is prone, but the appropriate time interval for examination has not been established. If the patient's head does not fit the headrest adequately (e.g., it is too large), consideration should be given to securing the head with a pin head holder. Some surgeons are now routinely placing the head in a pin head holder, which eliminates any opportunity for pressure on the eyes, but its use must be weighed against the associated risks. For most procedures in which the patient is prone, I recommend any of the commercially available square foam headrests. The head is positioned straight down in the neutral position. The eyes and nose are then placed in the open portion of the headrest, and the anesthesiologist may easily reach underneath to check for pressure intermittently. It is also important to ensure that the endotracheal tube, temperature probe, and wires such as those connected to electrocardiographic leads are positioned so that they have no contact with the eyes.
In some patients, especially during lumbar spine procedures, the prone position may be used in combination with a Trendelenburg (head-down) orientation to improve surgical exposure and decrease venous bleeding at the operative site. This position could predispose to increased venous pressure in the head and could theoretically decrease retinal perfusion pressure. However, it is not known to what extent IOP is altered under these conditions. Nonetheless, when Trendelenburg positioning is combined with deliberate hypotension and the
In nasal and sinus surgery, the most important principles are avoidance of inadvertent injections into or compromise of the ocular circulation, as explained previously. Embolization during cardiopulmonary bypass remains a cause of retinal vascular occlusion. Better means of detecting and preventing this complication are needed. However, some surgical maneuvers may lower the incidence of arterial emboli.[158]
|
|
|
|
|
|
|
|
|
|
|
|
|