|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Perimetry is used for precise evaluation of the visual field. The test requires a fully cooperative patient and would not generally be useful in the immediate postoperative period. Visual fields can be approximated manually, but more sophisticated automated testing is typically available to better delineate the field deficit and monitor its progression. In AION, the lesion is often altitudinal (i.e., affecting the superior or inferior part of the visual field), sometimes combined with circumferential constriction of the visual field. The measured overall visual acuity may be unaffected. This restriction in vision reflects a watershed infarction in the distribution of the paraoptic PCAs combined with edema of the ON in the region of the lamina cribrosa. Any edema in this region would result in damage to the axons of the ON as they exit this region.[14] [52] [53] In CRAO, the eye is usually blind initially. In BRAO, the visual loss is sectorial and corresponds to the area supplied by the occluded arterial segment.
CT and MRI are important in determining the extent of infarction of the brain associated with cortical blindness.
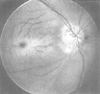 Figure 82-7
Funduscopic appearance of retinal vascular occlusion.
Note the pallor of the retina and the cherry-red spot, the clinical hallmark of
retinal arterial occlusion, visible in the fovea near the center of the picture.
The ischemic retina loses its normal transparency, and because the fovea is thinner
than the surrounding retina, the underlying choroid is visible as a cherry-red spot.
(From Ryan SJ: Retina, ed 2. St Louis, CV Mosby, 1995.)
Figure 82-7
Funduscopic appearance of retinal vascular occlusion.
Note the pallor of the retina and the cherry-red spot, the clinical hallmark of
retinal arterial occlusion, visible in the fovea near the center of the picture.
The ischemic retina loses its normal transparency, and because the fovea is thinner
than the surrounding retina, the underlying choroid is visible as a cherry-red spot.
(From Ryan SJ: Retina, ed 2. St Louis, CV Mosby, 1995.)
Orbital MRI or CT may also be helpful in diagnosis of ION, where enlargement of the ONs from edema is sometimes evident in the acute period.[55] [56] [57] Small, atrophic ONs may be visible with MRI in later stages of the disease.[58] However, MRI is frequently nondiagnostic. With CRAO and BRAO, MRI of the ON is normal; extraocular muscle damage might be evident in the setting of prolonged or severe compression of the eye.
The electroretinogram (ERG) and visual evoked potentials (VEP) can distinguish retinal from ON lesions. The full-field flash ERG is the most widely used.[59] A contact lens electrode is placed on the cornea and a reference electrode on the conjunctiva or the skin. The characteristic response is a small, early negative a wave and a larger, immediately following positive b wave. This "mass" response is a sensitive indicator for the presence of retinal injury, but it may not be able to precisely localize the retinal cell layers affected. The ERG is normal in ION, whereas the b wave is depressed after CRAO with irreversible ischemic damage ( Fig. 82-9 ). ION would not typically result in an abnormal ERG recording unless there was damage to the retinal ganglion cells or choroidal ischemia.[60]
The VEP records the response to flash or checkerboard pattern stimuli presented in front of the eye and is recorded with electrodes over the occipital cortex. Abnormal VEP responses suggest pathology distal to the orbit, that is, the ON and its projections to the brain, or the occipital cortex. Therefore, an abnormal VEP and a normal ERG would suggest a diagnosis of ION, not RAO.
In performing fluorescein angiography, 5 mL of sodium fluorescein dye is injected into an antecubital vein and is distributed within minutes throughout the body. The fluorescein is excited by light at certain frequencies, and the light emitted after excitation can be visualized in the eye by high-speed fundus photography. In recent years, digital video imaging has been used to enhance analysis.[61] The retinal and the choroidal circulations are separated by the pigment epithelium and can be visualized simultaneously and distinguished by three-dimensional stereo images. A distinct temporal perfusion pattern can be noted, with the choroid filling before the retina.[60] In AION, both the onset and completion of optic disk filling are delayed ( Fig. 82-10 ).[62] CRAO and BRAO produce characteristic perfusion deficits in the retinal circulation. However, the results of follow-up angiography have little prognostic value.[51]
Ultrasound uses a high-frequency (7.5 MHz) transducer held over the eye. Two-dimensional (B-mode) imaging is useful for visualization of gross ocular anatomy, whereas one-dimensional (A-mode) imaging gives more detailed information on the thickness and reflectivity of ocular structures.[60] Combining A- and B-mode imaging may enable evaluation of fluid in the ON sheath (e.g., in ION).[63]
CRAO/BRAO and ION both produce abnormal pupillary responses; cortical blindness does not. CRAO/BRAO and ION can generally be distinguished by findings on ophthalmoscopy. CRAO/BRAO may produce retinal pallor and a cherry-red spot; ION is characterized by optic disk edema or a normal disk initially and optic atrophy later. CT and MRI are useful for delineating the area of cerebral cortex damaged in cortical blindness, and orbital MRI may detect ON or extraocular muscle damage.
|
|
|
|
|
|
|
|
|
|
|
|
|