|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Normally, the kidneys receive 1000 to 1250 mL/min of blood in the average adult. This amount far exceeds that needed to provide the kidney's intrinsic oxygen requirement but ensures optimal clearance of all wastes and drugs. Essentially, all blood passes through glomeruli, and about 10% of renal blood flow is filtered (i.e., GFR of 125 mL/min in the normal adult). The basal normal blood flow is 3 to 5 mL/min/g of tissue, greater than in most other organs (i.e., seven to eight times as much as basal coronary blood flow and 400 times as much as skeletal muscle blood flow). This average primarily reflects blood flow in the cortical glomeruli because perfusion to the inner medulla and papilla is only about one tenth of total flow.
The vascular structure of the renal cortex is complex, and intracortical blood flow may not be evenly distributed[155] ( Fig. 37-9 ). The renal artery enters the kidney at the hilum, where it divides into five interlobar arteries, each an end artery. At the junction of the renal medulla and cortex, the interlobar branches divide into arcuate arteries, which course at right angles to the interlobar arteries. The interlobular arteries arise at right angles to the arcuate arteries and penetrate through the renal cortex. The afferent arterioles, which arise from the interlobular arteries, divide within the cortical tissue to form the glomerular capillary network. The capillaries then reunite to form the efferent arterioles. The subsequent course of the efferent arterioles depends on whether they are located superficially in the cortex or are situated in the juxtamedullary cortex. Superficial efferent arterioles feed a plexus of peritubular venous capillary vessels. These vessels supply the proximal and distal tubules and portions of the loops of Henle and the collecting ducts before joining the interlobular veins and returning to the inferior vena cava through the arcuate, interlobar, and renal veins. Juxtamedullary efferent arterioles also supply the venous capillary network and give rise to the vasa recta, small-diameter vessels that penetrate deep into the medulla. These vessels provide most of the oxygen supply to the loop of Henle and join together to form the arcuate veins. The juxtaglomerular apparatus is between the afferent and efferent arterioles and the macula densa, a specialized group of cells in the distal convoluted tubule.
Because the renal cortex contains most of the glomeruli and depends on oxidative metabolism for energy, ischemic hypoxia injures the renal cortical structures, particularly the pars recta of the proximal tubules. As ischemia persists, the supply of glucose and substrates continues to decrease; glycogen is consumed, and the medulla, which depends to a greater extent on glycolysis for its energy sources, becomes more adversely affected. Interruption of blood flow to kidneys for more than 30 to 60 minutes results in ARF and irreversible cell damage. Early cell changes are reversible, such as the swelling of cell organelles, especially the mitochondria. As ischemia progresses, lack of adenosine triphosphate interferes with the sodium pump mechanism, water and sodium accumulate in the endoplasmic reticulum of tubular cells, and the cells begin to swell. In irreversible ARF, the several pathologic changes occur. Swelling of tubular epithelial cells leads to formation of bullae, which protrude into the tubular lumen distal to the cell; necrosis of tubular cells results in abnormal membrane permeability; structural changes in the glomerular epithelium may decrease glomerular filtration; and constriction of intrarenal arteries and arterioles may further reduce glomerular blood flow.
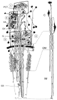 Figure 37-9
Diagram demonstrating the complex vascular and tubular
organization of the kidney. The pattern of glomeruli (G) arising from afferent arterioles
(AA) is demonstrated in the left-hand portion of the figure. Proximal convoluted
tubules (PCT) are perfused by a peritubular capillary network derived from efferent
vessels (EV). Loops of Henle are grouped with collecting ducts (CD). Only thin
limbs of Henle extend with collecting ducts to the papillary tip. These are accompanied
by vasa recta extending from the cores of the vascular bundles. C, cortex; OM, outer
medulla; IM, inner medulla. (From Beeuwkes R, Bonventre JV: Tubular organization
and vascular tubular relations in the dog kidney. Am J Physiol 229:695–713,
1975.)
Figure 37-9
Diagram demonstrating the complex vascular and tubular
organization of the kidney. The pattern of glomeruli (G) arising from afferent arterioles
(AA) is demonstrated in the left-hand portion of the figure. Proximal convoluted
tubules (PCT) are perfused by a peritubular capillary network derived from efferent
vessels (EV). Loops of Henle are grouped with collecting ducts (CD). Only thin
limbs of Henle extend with collecting ducts to the papillary tip. These are accompanied
by vasa recta extending from the cores of the vascular bundles. C, cortex; OM, outer
medulla; IM, inner medulla. (From Beeuwkes R, Bonventre JV: Tubular organization
and vascular tubular relations in the dog kidney. Am J Physiol 229:695–713,
1975.)
Onset of tubular damage usually occurs within 25 minutes of ischemia as the microvilli of the proximal tubular cell brush borders begin to change. Within an hour, they slough off into the tubular lumen, and membrane bullae protrude into the straight portion of the proximal tubule. After a few hours, intratubular pressure rises, and tubular fluid backflows passively. Within 24 hours, obstructing casts appear in the distal tubular lumen. Even if renal blood flow is completely restored after 60 to 120 minutes of ischemia, the GFR may not immediately improve.[64]
In ischemia-induced ARF, lesions are unevenly distributed among nephrons, probably reflecting variability in blood flow. In nephrotoxin-induced failure, the necrosis of proximal tubular cells obstructs the tubular lumen with castlike material, and lesions are more evenly distributed among all nephrons.[156] After restoration of blood flow, intrarenal vascular resistance is high because of cell swelling and ischemic damage, and flow is as much as 50% below control values. [64] Renal hypoperfusion in the setting of a toxic insult may act in synergy to increase the risk of ARF. As blood flow becomes inadequate to support glomerular filtration, urine flow ceases, and toxic substances accumulate in the urine and renal parenchyma. Other factors contributing to increase the risk of renal failure include the interference of certain drugs (e.g., nonsteroidal anti-inflammatory drugs) with autoregulation and preservation of blood flow to strategic regions of the kidney, drug-induced membrane damage, or mitochondrial uncoupling, causing an insufficient use of oxygen. Toxic substances (e.g., aminoglycosides) and oxygen deprivation because of low blood flow produce depletion of adenosine triphosphate.
Renal function reflects and is reflected by regional nephron heterogenicity and changes in regional renal blood flow. The vascular pattern of the kidney is complex, and the distribution of renal blood flow is nonhomogeneous (see Fig. 37-9 ). In the clinical setting of hypotension, the kidney appears to have a distinct susceptibility to injury. The reason for this susceptibility is not readily apparent, because renal blood flow is normally high and oxygen supply exceeds by far the requirements for oxygen use. This overall balance, however, may conceal regional hypoxia, predominantly in the outer medulla.
The interest in renal blood flow as a predictor of renal function was first stimulated in 1947, when Trueta and associates[157] introduced the concept of cortical ischemia and attributed the pathogenesis of ARF to increased blood flow through the medulla. Supporting evidence for this view has since been offered by many investigators who measured renal blood flow distribution with various methods.[158] [159] [160] [161] Monitoring regional renal blood flow patterns may help us understand the physiology of renal salt and water excretion and the comparative pharmacologic effects on the so-called redistribution of blood flow within the cortex. Perhaps nephrons in the outer cortex are "salt losers," and those in the inner cortex are "salt retainers." If this is true, salt-retaining states should occur with the selective reduction of renal blood flow to the superficial cortex, and these states may or may not be accompanied by a rise in renal blood flow to the deep cortex.[30] [161]
Because oliguria most commonly results from reduced cardiac output caused by hypovolemia, it is essential to understand how hypovolemia affects distribution of renal blood flow. Activation of the sympathetic nervous system and possibly the renin-angiotensin system reduces renal blood flow and the GFR. Despite well-maintained
Renal arteriographic and xenon washout studies in patients with ARF have shown selected, profound reduction in renal blood flow.[160] Arteriography revealed severe attenuation of the intrarenal arterial tree, inability to visualize cortical vessels, absence of a normal cortical nephrogram, and striking reduction in the velocity of contrast dye as it passed through the kidney. These same patients had no bleeding from the cortex during open renal biopsy. The xenon washout studies also show that the usual rapid transit of xenon through the cortex (indicating cortical perfusion) in the normal kidney is absent in acute oliguria. Renal cortical perfusion at one third of its normal level, involving constriction of the afferent arterioles, is a condition that is more than sufficient to stop renal functioning.
The understanding that distribution of renal blood flow is nonhomogeneous, the recognition of nephron structure and functional heterogenicity, and the suggestion that changes in the zonal distribution of renal blood flow affect renal salt and water homeostasis have led investigators to focus on developing new techniques to measure intrarenal blood flow.[161] Methods to measure distribution of renal blood flow in humans include the clearance of para-aminohippurate, indicator dilution, renocortical tissue Po2 , radiolabeled tracers, Doppler ultrasonography, and external gas-washout techniques. The risks and limitations associated with these techniques limit their usefulness.
Any nontoxic substance cleared by the kidney and not metabolized
may be used to measure renal blood flow by application of the Fick principle, whereby
renal plasma flow is calculated as follows:
RPF = UV/(A − RV)
U is urinary concentration, V is urine flow rate, A is arterial plasma concentration,
and RV is renal venous plasma concentration. Renal blood flow (RBF) is then calculated
from the following formula:
RBF = RPF/(1 − Hematocrit)
The volume of plasma from which the kidney can extract and excrete a specific substance
in a given time is the renal clearance of the substance. If a substance is completely
eliminated from the plasma in one pass through the renal circulation, is filtered
at the glomerulus, is not synthesized or destroyed, and is physiologically inert,
it would be ideal for measuring renal plasma flow. Para-aminohippurate
is a substance that almost meets these criteria. The practical advantage of measuring
the extraction of para-aminohippurate is that its
renal extraction is approximately 90% in humans after one passage. Measurement of
renal blood flow is based on the assumption that cortical extraction is 100% and
that extraction in the medulla is 0%; therefore, it has been assumed that the nonextracted
fraction (10%) should reflect the fraction of blood flowing through the medulla.
However, experimental evidence shows that cortical extraction is not 100%,[162]
casting doubt on the accuracy of intrarenal blood flow assessment with extraction
techniques. Other limitations of the extraction technique include the requirement
for a high rate of urine flow, a steady state for 15 to 30 minutes, and the sampling
of systemic arterial and renal venous blood.
Indocyanine green, which is bound to albumin in plasma, has been used in the indicator dilution method with densitometry. The indicator dilution method necessitates catheterization of a renal artery and vein. Although attempts have been made to measure renocortical (fast) and medullar (slow) blood flows separately from dye dilution curves, no convincing results have been produced.[163] Measurement of renal blood flow by a continuous thermodilution technique, which requires renal venous cannulation and continuous injection of normal saline, has been proposed.[164] Measurements have correlated with calculated renal blood flows derived from clearance techniques in patients after cardiac catheterization; however, only total renal blood flow is measured with the thermodilution technique.
Tissue oxygen pressure is measured with a multiwire surface PO2 electrode and temperature probe. This polarographic Clark-type electrode has multiple platinum microelectrodes arranged in an array in the center of the tissue contact area, with a polarographic silver anode positioned at its circumference. The probe is placed directly on the renal cortex and measures changes in local tissue PO2 , which is an indirect indicator of renal surface perfusion.[165] [166] [167] However, this device measures only outer cortical renal blood flow changes. Because ischemic insults to the kidney are associated with a marked decrease in blood flow to the outer cortex, a redistribution of blood flow to the juxtamedullary glomeruli in the inner cortex and medulla is presumed.
External krypton 85 and xenon 133 clearance techniques estimate local blood flow per gram of tissue from local gas clearance.[168] The gas-washout technique requires selective catheterization of the renal artery and is usually performed in conjunction with arteriography. The renal artery also may be punctured intraoperatively for injection. After intra-arterial injection, the gas diffuses rapidly into the renal tissue, theoretically equilibrating in tissue and blood. The renal washout curve is then assessed by external counting ( Fig. 37-10 ). The reliability of washout methods has been questioned, and absolute flow rates cannot be determined with this method. In anuric subjects, various causes for unreliability of the method may be suspected. For example, the disappearance rate of the gas decreases when kidney volume increases, and kidney volume typically increases during ARF. The partition coefficient of 133 Xe may be less than 1.0 when the water content of the kidney is
 Figure 37-10
Analysis of an externally monitored radioactive krypton
washout curve for the kidney in a dog. Notice the logarithmic vertical scale. The
rapid component (I) represents cortical blood flow. Half-times for each component
together with estimated flow rates and compartment sizes are shown in the table above
the plotted curves. (From Thorburn GD, Kopald HH, Herd A, et al: Intrarenal
distribution of nutrient blood flow determined by krypton 85 in the unanesthetized
dog. Circ Res 13:290, 1963.)
Figure 37-10
Analysis of an externally monitored radioactive krypton
washout curve for the kidney in a dog. Notice the logarithmic vertical scale. The
rapid component (I) represents cortical blood flow. Half-times for each component
together with estimated flow rates and compartment sizes are shown in the table above
the plotted curves. (From Thorburn GD, Kopald HH, Herd A, et al: Intrarenal
distribution of nutrient blood flow determined by krypton 85 in the unanesthetized
dog. Circ Res 13:290, 1963.)
Qualitative and semi-quantitative evaluation of renal perfusion can be obtained with a gamma camera recording of the transit of a radiopharmaceutical tracer through the kidney. Qualitative evaluation of renal perfusion consists of the visual assessment of the serial images and comparative assessment of the first transit of tracer from the aorta (or iliac-renal artery) to the kidney. Among the limitations associated with the renogram is movement of the radiopharmaceutical (injected intravenously as a bolus) before it reaches the kidney, necessitating that the pharmacokinetic model contain all the possible exchange compartments between the kidney and the plasma for accurate analysis of the tissue radioactivity curve. The presence of low urine flow rates, dilated pelvic caliceal cavities, or severely reduced renal function may also cause difficulties for test interpretation. In general, the information that can be derived from the renogram includes renal clearance (as a fraction of the radioisotope to blood volume) and blood flow of the two kidneys relative to each other.[170] Because the results obtained are comparative, lesions are revealed only when they have asymmetric distribution.
Duplex ultrasound has enabled evaluation of changes in renovascular resistance and intrarenal blood flow in patients. The technique is noninvasive and may be repeated as often as required. Typically, an interlobar artery is selected for evaluation for several reasons. The anatomic course and relationships of the vessel allow easy recognition on subsequent serial scans in the same patient; the vessel is large enough to assume laminar flow throughout its length; and the angle of the ultrasound beam can generally be minimized to assume maximum Doppler frequency shift. Renal vascular resistance can be assessed by the pulsatility index.[171] The lower the pulsatile index, the less resistance there is to flow. The value is derived by dividing the difference between systolic maximum height and diastolic minimum height of the waveform by its mean height ( Fig. 37-11 ). Change in flow velocity can be assessed from a change in the mean frequency shift. The frequency shift is proportional to the mean velocity of blood flow within the vessel multiplied by the cosine of the angle between the ultrasound beam and the direction of flow. The angle is assumed to be close to zero. With this method, only blood flow velocity in large interlobar arteries and resistance can be assessed. The duplex Doppler ultrasound technique may provide a unique opportunity to demonstrate the effects of drugs on the renal vasculature and to predict the onset of pending renal failure or transplanted kidney rejection. Overall, this technique should provide an opportunity for measuring relative changes in large vessel flow velocity but not in absolute renal blood flow.
Contrast ultrasonography has been used to image renal blood flow. [172] [173] [174] [175] The microbubbles produced by ultrasonic cavitation (i.e., sonication) are smaller than red blood cells and, in passing with them through the
 Figure 37-11
Renal duplex scan shows a sample volume superimposed
over a B-scan image for a typical normal velocity flow pattern from the main renal
artery at the hilum. theta, angle of incidence between transmitted ultrasound
signal and received ultrasound signal; vd, diastolic velocity; vS, systolic velocity.
Figure 37-11
Renal duplex scan shows a sample volume superimposed
over a B-scan image for a typical normal velocity flow pattern from the main renal
artery at the hilum. theta, angle of incidence between transmitted ultrasound
signal and received ultrasound signal; vd, diastolic velocity; vS, systolic velocity.
|
|
|
|
|
|
|
|
|
|
|
|
|