Biochemical Markers of Renal Function
The kidney contains many different cell types, and the primary
roles are glomerular filtration, tubular reabsorption and secretion, and concentration
of metabolites. Although complex, the kidney may be able to respond to
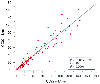 Figure 37-8
Correlation between the 2-hour creatinine clearance (CC02)
and the 22-hour creatinine clearance (CC22). (Adapted from Sladen RN, Endo
E, Harrison T: Two-hour versus 22-hour creatinine clearance in critically ill patients.
Anesthesiology 67:1013, 1987.)
Figure 37-8
Correlation between the 2-hour creatinine clearance (CC02)
and the 22-hour creatinine clearance (CC22). (Adapted from Sladen RN, Endo
E, Harrison T: Two-hour versus 22-hour creatinine clearance in critically ill patients.
Anesthesiology 67:1013, 1987.)
disease in only a limited number of ways at the cellular level. Noninvasive tests
have been developed that reflect the morphologic, functional, and biochemical regional
specialization.
NAG is a widely used urinary enzyme assay for the assessment of
renal disease and the detection of nephrotoxicity. Unlike a number of unstable enzymes
excreted into the urine, NAG remains suitable for clinical diagnosis of renal disease.
Increased NAG activity detected in urine is a sensitive test for renal tubular damage.
[140]
Its molecular mass precludes filtration by
the glomerulus, and it is neither absorbed nor secreted by the tubules. It is also
the most active glycosidase found in the proximal tubule lysosomes. Any increase
in urinary concentration of NAG may be considered a marker for tubular damage. The
value of NAG as a diagnostic test is further enhanced by its presence in a number
of isoenzyme forms. The relative amount of each isoenzyme varies at different stages
of renal disease.
A number of analytical methods are available for the determination
of urinary NAG, including fluorometric, colorimetric, spectrophotometric, and dipstick
tests. Each of these methods is tedious and is associated with limitations that
prevent widespread clinical adaptation. Low levels of NAG are excreted by normal
individuals, and assay procedures must be sensitive enough to overcome the endogenous
inhibitor urea.[141]
Another factor to overcome
is variation from urine collection that occurs over time. Factoring the enzyme activity
with creatinine concentration over the same urine flow collection period is a reasonable
approach for this problem.[142]
In general, the
sensitivity and reproducibility of the fluorometric method, when it is performed
correctly, is excellent, but the equipment necessary is not commonly available in
laboratories.
The colorimetric technique overcomes the limitations of the fluorometric
technique by the incorporation of a calibrant for easy interlaboratory comparison
and access in most clinical chemistry laboratories, with modification for use with
spectrophotometric analysis.[143]
The latest development
is a dipstick method for detection of NAG in the urine.[144]
The NAG strip incorporates a biochemical derivative that releases a blue-violet
color on hydrolysis, and the test requires up to 30 minutes after the addition of
reagents. However, problems can arise if the sample is contaminated with blood or
bilirubin. Besides pigmented material in urine, a concentrated urine specimen that
is high in urea also renders the test inaccurate.
Perhaps the most interesting discovery regarding urinary NAG for
detection of renal disease is that there appears to be isoenzyme specificity for
various types of pathology.[145]
NAG is the most
active lysosomal hydrolase and is normally found in tissues as two major forms:
A and B. These major forms differ in their subunit composition. Traditionally,
the main clinical interest in these isoenzymes had been their use in the detection
of two autosomal recessive disorders, Tay-Sachs disease and Sandhoff disease. In
1970, Price and coworkers[146]
reported that the
B form increased in a urinary pattern of NAG after surgical trauma. Since that time,
it has been appreciated that the relative amount of the B form increased (i.e., the
ratio of A to B forms decreased) compared with urine in the normal population. Automated
methods for separation of NAG isoenzymes allow the pattern of excretion to be compared
in various disease states ( Table 37-6
).
Of interest to the anesthesiologist is that evidence suggests that after major surgery,
the percentage of an intermediate form (I) increases in the urine.[147]
Smaller increases in the I form were also observed in rejection of renal transplants.
Rejection was more strongly associated with a decrease in the A/B ratio in a cohort
of renal transplant patients. No change in the isoenzyme profile was found in stable
transplant patients, whereas reversible rejection was characterized by an increase
in the I form and a decrease in the relative amount of the A form present. When
a patient did not respond to treatment, the I and the B forms were elevated, but
levels of the A form decreased.[148]
Different nephrotoxic drugs or conditions appear to produce characteristic
urinary isoenzyme profiles. For example, the B and I forms are elevated after administration
of aminoglycosides. Total urinary and serum NAG activity also has been reported
to increase in diabetic patients. Overall, NAG activity in the urine reflects the
activity of the disease or severity of the damage. Serial monitoring is therefore
most useful because trending is the most appropriate method to interpret the results.
The NAG-to-creatinine ratio is a more sensitive and specific marker for renal tubular
dysfunction. It is useful to express NAG as a ratio of urinary creatinine to minimize
dilutional or concentration effects. The lack of sensitive, simple, inexpensive,
and efficient methods is the limiting factor for widespread clinical use of NAG monitoring.
Another urinary enzyme, clusterin, may prove to be more specific than NAG[149]
for evaluating nephrotoxicity caused by aminoglycoside use while remaining equally
sensitive.
Several other urinary constituents have been identified to detect
cytotoxic and abnormal processes in specific regions of the kidney ( Table
37-7
). α-Glutathione S-transferase
is found principally in the proximal convoluted tubules, and π-glutathione S-transferase
is found principally in the distal convoluted tubule. β2
-Microglobulin
is a subunit of the class I antigen of the
TABLE 37-6 -- Variation of urinary N-Acetyl-β-D-Glucosaminidase
|
Concentration of Isoenzyme
Form |
|
Condition |
A |
I |
B |
|
Severe renal damage |
↓ |
↑ |
↑ |
|
Major surgery |
↓ |
— |
↑ |
|
Reversible renal transplant rejection |
↓ |
↑ |
— |
|
Nonreversible renal transplant rejection |
↓ |
↑ |
↑ |
|
Aminoglycoside administration |
— |
↑ |
↑ |
|
↑, increased; ↓, decreased; —, no change. |
|
From Campbell JAH, Conigall AV, Guy A, et al: Immunohistologic
localization of alpha, mu, and pi class glutathione S-transferases in human tissue.
Cancer 67:1608–1613, 1991. |
TABLE 37-7 -- Urinary constituents used to measure renal function
|
Glomerular permeability and selectivity |
Albumin |
|
Transferrin |
|
Aspartate aminotransferase |
|
Immunoglobulin G |
|
Tubular protein uptake |
β2
-Microglobulin |
|
Retinol binding protein |
|
Ribonuclease |
|
Proximal tubular brush border |
Alanine aminopeptidase |
|
γ-Glutamyl transpeptidase |
|
Proximal tubule |
N-Acetyl-β-D-glucosaminidase |
|
α-Glutathione S-transferase |
|
Thick ascending limb |
Tamm-Horsfall protein |
|
Distal tubule |
π-Glutathione S-transferase |
|
From Baines AD: Strategies and criteria for developing
new urinalysis tests. Kidney Int Suppl 47:S137–S141, 1994. |
major histocompatibility complex and is structurally homologous to immunoglobulins.
[150]
Its mass is 11,600 daltons, it is freely
filtered
from plasma by the renal glomerulus, and more than 99.9% is reabsorbed in the proximal
tubule. β2
-Microglobulin is measured by radioimmunoassay and immunodiffusion
techniques.[151]
Its secretion in urine is a sensitive
indicator of tubular damage or disease. Limitations to its use include its unstable
nature in urine of pH 5.5 or below, thereby precluding its use for patients with
concomitant urinary tract infection or pyuria. Its degradation by proteolysis is
affected by temperature, and its measurement requires a sophisticated laboratory
that precludes widespread clinical use. α1
-Microglobulin is filtered
at the glomerulus and is 95% reabsorbed at the proximal tubule, thereby indicating
proximal tubular dysfunction when present in the urine.[152]
[153]
[154]
Alanine
aminopeptidase excretion has been identified as a specific marker for proximal tubular
brush border dysfunction, as has γ-glutamyl transpeptidase excretion. Plasma
and urinary cytokines, such as interleukin-8, interleukin-10, interleukin-1 receptor
antagonist, and tissue necrosis factor soluble receptor-2, have been shown to correlate
with proximal tubular dysfunction.[152]
In general,
enhanced excretion of biochemical markers, tubular enzymes, or antigens may be the
consequence of an exfoliated damaged tubular cell, an increased turnover of tubular
cells, or some other metabolic disturbance. The biologic variability of the sensitive
analytes in response to physiologic stress is large compared with anticipated changes
observed in early disease. The signals of early, irreversible disease may not always
be distinguished from the noise of biologic variability, and use of these markers
should be applied with appropriate caution.
 Figure 37-8
Correlation between the 2-hour creatinine clearance (CC02)
and the 22-hour creatinine clearance (CC22). (Adapted from Sladen RN, Endo
E, Harrison T: Two-hour versus 22-hour creatinine clearance in critically ill patients.
Anesthesiology 67:1013, 1987.)
Figure 37-8
Correlation between the 2-hour creatinine clearance (CC02)
and the 22-hour creatinine clearance (CC22). (Adapted from Sladen RN, Endo
E, Harrison T: Two-hour versus 22-hour creatinine clearance in critically ill patients.
Anesthesiology 67:1013, 1987.)