|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Although the kidneys constitute less than 0.5% of body mass, they receive 20% to 25% of the total cardiac output in the normal adult. The distribution of cardiac output to the kidneys may be influenced by a number of factors, including hypovolemia, activation of the sympathetic nervous system or the renin-angiotensin system, extrinsically administered vasopressors and vasodilators, and local neurohumoral factors.
The fraction of cardiac output perfusing the kidneys depends on the ratio of renal vascular resistance to systemic vascular resistance.[18] In general, the response to renal hypoperfusion involves three major regulatory mechanisms that support renal function: afferent arteriolar dilation increases the proportion of cardiac output that
The kidney produces vasodilator prostaglandins to counteract the effects of systemic vasoconstrictor hormones such as angiotensin II.[100] In a state of low cardiac output when systemic blood pressure is preserved by the action of systemic vasopressors, renal blood flow is not depressed because the effect of the vasopressors is blunted within the kidney.
A selective increase in efferent arteriolar resistance decreases glomerular plasma flow, thereby preserving GFR. Glomerular filtration is augmented because capillary pressure upstream from the site of vasoconstriction tends to rise. This mechanism enables the kidney to offer high organ vascular resistance to contribute to the maintenance of systemic blood pressure without compromising its function of filtration. Studies using specific inhibitors of angiotensin II have shown that efferent arteriolar resistance largely results from the action of angiotensin II. [101] At low concentrations, norepinephrine has a vasoconstricting effect on efferent arterioles, indicating that the adrenergic system may also be important for maintaining the renal compensatory response.[102]
There is abundant evidence to support the notion that reductions in cardiac output are accompanied by the release of vasopressin and by increased activity of the sympathetic nervous system and the renin-angiotensin-aldosterone system. These regulatory mechanisms to preserve renal blood flow conserve salt and water. The control of blood delivery to the kidney, the fraction of plasma filtered, and the amount of volume returned to the systemic circulation are determined by regulatory mechanisms within the kidney that attempt to preserve filtration function during compromised circulation. These compensatory mechanisms, however, have limits. Excessive vasoconstrictive forces may eventually induce a decrease in filtration function. This shift from compensation to decompensation may be exacerbated by pharmacologic interventions. Oliguria is both a symptom of ARF and a consequence of the normal compensatory mechanisms to prevent it. Differentiating between the two is critical for outcome and therapeutic strategies.
Clinical investigators have expended considerable effort to evaluate laboratory tests of serum and urinary indices that attempt to differentiate the cause of oliguric states, to predict outcome, and to direct therapy for the prevention of ARF. Unfortunately, none of the tests is sensitive or specific enough to predict which patients will or will not respond to therapy. Among the tests commonly obtained are urine volume, urine specific gravity, urine osmolality, serum creatinine level, serum GFR level, urine-to-plasma creatinine ratio, urine-to-plasma urea ratio, urine sodium level, fractional excretion of sodium, free rate clearance, and creatinine clearance.
Monitoring urinary output as a sign of the adequacy of renal perfusion is easy and is based on the assumption that patients with diminished renal perfusion excrete a low volume of concentrated urine. Urinary flow rate is, however, an indirect parameter of renal function because many nonrenal factors may directly and profoundly affect urine output. Oliguria (anuria) during hypotesive anesthesia on one hand and polyuria on the other demonstrate that low urine output does not specifically reflect inadequate renal perfusion and that normal urine output does not guarantee a normally functioning kidney. The presence of urine flow (regardless of the amount) indicates that there is blood flow to the kidney, because without perfusion, glomerular filtration and generation of urine cease. The level of nitrogenous wastes in the blood depends on systemic production and renal clearance. Renal clearance depends on renal blood flow and glomerular filtration. The kidney concentrates the ultrafiltrate so that at maximal concentration, 400 to 500 mL of urine is required to clear the daily obligatory nitrogenous wastes.[103] Traditionally, inadequate urinary volume, or oliguria, is defined as urinary output of less than 0.5 mL/kg/hr. Multiple studies, however, have shown no correlation between urine volume and histologic evidence of acute tubular necrosis, GFR, creatinine clearance, or changes from preoperative to postoperative levels of BUN and creatinine levels in patients with burn injury,[104] trauma,[105] [106] or shock status or in those under-going cardiovascular surgery.[107] [108] [109] Moreover, measurement of mean and lowest hourly urinary output during aortic vascular surgery has not been predictive of postoperative renal insufficiency.[107]
Oliguria may reflect a variety of factors independent of GFR, and a normal hourly urine output does not rule out renal failure. Among these factors is tubular excretion of solute and water, which is determined by local and systemic levels of renin, aldosterone, and ADH. In the operating room, patients are often hemodynamically unstable; decreased blood volume or cardiac output, fluctuating hormone levels (e.g., aldosterone, renin, ADH), nervous system reflexes, and increased catecholamine concentrations, added to the effects of general anesthesia, can alter GFR. The data do not support oliguria as a reliable sign of pending renal dysfunction.[103] [104] [105] [106] [107] [108] [109]
The specific gravity of urine is also easy to measure. In this test, the mass of 1 mL of urine is compared with the mass of 1 mL of distilled water to reflect renal concentrating ability. With poor perfusion or prerenal azotemia, urine specific gravity is 1.030, reflecting the kidney's conservation of sodium and water. With acute tubular necrosis, the loss of concentrating ability moves urine
Osmolality is a measure of the number of osmotically active particles in solution in the solvent phase. It is one of the major forces that move fluid throughout the body, especially in the kidney. Theoretically, urine osmolality is physiologically superior to urine specific gravity as a test of renal function; however, the same substances and conditions that render urine specific gravity a nonspecific test can also affect the reliability of urine osmolality (see Table 37-4 ). Since the late 1940s, physicians have recognized the inability of the kidneys to produce concentrated urine during acute tubular necrosis and have used as a guideline urine osmolality of greater than 500 mOsm/kg H2 O to identify prerenal azotemia and urine osmolality of less than 350 mOsm/kg H2 O to indicate acute tubular necrosis. A defective urinary concentrating mechanism tends to be one of the most consistent and lasting tubular defects of ARF.
The sensitivity and specificity of urine osmolality as a test
for predicting or distinguishing acute tubular necrosis from prerenal azotemia are
clinically inadequate.[109]
[112]
[113]
[114]
[115]
[116]
[117]
With
values greater than 500 mOsm, the positive predictive value for diagnosing prerenal
azotemia ranges from 60% to 100%. With a value less than 350 mOsm, the positive
predictive value for diagnosing acute tubular necrosis ranges from 69% to 95%. The
measure of urine
| Proteins |
| Glucose |
| Mannitol |
| Dextran |
| Diuretics |
| Advanced age |
| Extremes of age |
| X-ray contrast media |
| Antibiotics (e.g., carbenicillin) |
| Hydrometer calibration |
| Detergents |
| Temperature |
| Hormonal imbalances |
The relationship between serum creatinine or BUN levels and GFR seems to make the determination of either one an ideal indicator of filtration rate. However, elevations of serum creatinine and BUN levels are late signs of renal dysfunction because the GFR must often be reduced as much as 75% before elevations reach abnormal levels,[118] and many nonrenal variables affect serum creatinine and BUN levels ( Table 37-5 ). A product of protein metabolism, BUN level is increased by high protein intake, blood in the gastrointestinal tract, and accelerated catabolism (e.g., in traumatized or septic patients). The normal range is 8 to 20 mg/dL. Because urea is synthesized in the liver, hepatic dysfunction decreases urea production and therefore BUN concentration. BUN concentration fails a key criterion as a reliable estimate of the GFR. Although urea is freely filtered at the glomerulus, it is reabsorbed to a large and variable extent. The reabsorption of urea is greater, approximately 60% of the filtered load, when urinary flow is low; only about 40% is reabsorbed when flow is high.
Creatinine, a product of skeletal muscle protein catabolism, is
produced at a lower rate in elderly than in young adults and in women than in men.
Consequently, serum creatinine levels may fail to accurately reflect the magnitude
of nephron loss. Because creatinine production is proportional to muscle mass, many
chronically ill, wasted, elderly patients have serum creatinine values in the normal
range, despite reduced renal concentrating ability and GFR. Conversely, many critically
ill patients at risk for developing ARF may have high metabolic rates because of
hyperalimentation, sepsis, or post-traumatic states. Higher metabolic rates mean
higher nitrogenous waste production requiring greater than average renal blood flow
and urine flow rates to maintain normal serum creatinine concentration. In patients
with ARF, edema may dilute serum creatinine concentrations. Because creatinine is
excreted by glomerular filtration and tubular secretion, as the GFR decreases, the
tubular secretion becomes a progressively more important fraction of creatinine excretion,
such that creatinine clearance overestimates the GFR by 50% to 100% if the true GFR
is less than 15 mL/min.[118]
[119]
Postoperative serial serum creatinine
| Increased nitrogen absorption |
| Tissue breakdown |
| Body mass |
| Diet |
| Activity |
| Hepatic disease |
| Diabetic ketoacidosis |
| Large hematoma |
| Gastrointestinal bleeding |
| Drugs or steroids |
The urine-to-plasma creatinine index was first introduced in 1950 by Bull and colleagues[121] as a method of evaluating patients with acute tubular necrosis. It was determined retrospectively that patients whose urine-to-plasma creatinine levels rose above 10 after having been below 10 were no longer at serious risk for "uncontrolled mineral and water loss." This index was introduced primarily as a prognostic aid and not as a diagnostic test.
The sensitivity and specificity of the urine-to-plasma creatinine index are unfortunately clinically unreliable for diagnosing acute tubular necrosis and prerenal azotemia.[122] Miller and coworkers [113] concluded that the index was not useful, except in extreme situations, because of significant overlap between groups. Espinel and Gregory[114] prospectively studied 87 patients (61 with acute tubular necrosis or prerenal azotemia) and concluded that the urine-to-plasma creatinine ratio might be a useful indicator of acute tubular necrosis if the ratio was less than 10 and that it might be an indicator of prerenal azotemia if the ratio was less than 40. However, a significant overlap in values was found for 33% of patients.
In 1959, Perlmutter and colleagues[123] proposed that the ratio between urine and serum urea nitrogen levels could differentiate prerenal azotemia from acute tubular necrosis. These investigators thought that a ratio of greater than 14 was associated with transient oliguria and that a ratio of less than 10 was associated with acute tubular necrosis. In 1965, Eliahou and Bata[112] reported that the urine-to-blood urea ratio might not be useful for diagnosis in all cases. Subsequently, Chisholm and associatates[124] confirmed that the urine-to-plasma urea ratio was often misleading in the diagnosis of uremia.
Urea production is not constant and is affected by many nonrenal variables[125] (see Table 37-5 ). Variables include sudden increases or decreases in protein intake, states of increased protein catabolism (i.e., trauma, infections, hyperthermia, or steroid therapy), gastrointestinal bleeding, and liver dysfunction. Because urea levels can vary tremendously independent of renal function, it is not surprising that knowing the urine-to-plasma urea ratio does not make it possible to differentiate between prerenal azotemia and acute tubular necrosis.
With decreasing perfusion, the normally functioning kidney conserves sodium and water. The factors affecting this phenomenon are numerous and include heterogenicity of nephrons, secretion of aldosterone, secretion of ADH, diuretic therapy, saline content of intravenous infusion solutions, sympathetic neural tone, and sodium-avid states (e.g., congestive heart failure, cirrhosis). It is traditionally accepted that a urinary sodium level of less than 20 mEq suggests prerenal azotemia and that a level of greater than 40 mEq indicates acute tubular necrosis.
The reported sensitivity and specificity of urinary sodium levels for diagnosis of acute tubular necrosis and prerenal azotemia suggest that the levels can be used for diagnosis of approximately one half of the patients with acute tubular necrosis.[109] [113] [114] [115] [121] [126] [127] Considering the complex physiology of sodium homeostasis, it is not surprising that levels of urinary sodium are unreliable indicators of prerenal azotemia or acute tubular necrosis. The test is nonspecific because a significant overlap exists between the diagnostic categories and because urinary sodium measurement tends to correlate more with volume and type of resuscitation fluid than with renal function.[106]
The fractional excretion of sodium was first described by Espinel.
[126]
It represents the fraction of sodium excreted
of that filtered by the kidneys. Handa and Morrin[122]
had previously described the renal failure ratio, which is also derived from the
excreted fraction of filtered sodium. The only difference between the two is that
in determining renal failure ratio, it is assumed that plasma sodium level is controlled
within fairly narrow limits and is constant. The fractional excretion of sodium
(FeNa) is calculated as follows:
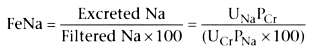
PCr
is the plasma creatinine level, PNa
is the plasma sodium
level, UCr
is the urinary creatinine level, and UNa
is the
urinary sodium level. All variables are easy to obtain.
At first investigators had reported that the fractional excretion of sodium clearly differentiated prerenal azotemia from acute tubular necrosis.[113] [114] [115] [126] Subsequently, case reports demonstrated that the fractional excretion of sodium was a nonspecific indicator and was not as sensitive as initially believed.[126] [127] [128] [129] [130] Brown[128] suggested that the fractional excretion of sodium was diagnostic of acute tubular necrosis but was of little or no value for the early prediction of renal failure. Brosius and Lau[129] showed that early determinations of the fractional excretion of sodium were more likely to be misleading than later determinations, indicating that the measurement was unlikely to be helpful in the early diagnosis of acute tubular necrosis. Shin and colleagues[105] concluded that knowing the fractional excretion of sodium was useful only when oliguria was already present. A sodium excretion value of less than 1% compared with greater than 1% enables differentiation between prerenal azotemia and acute tubular necrosis in most cases. However, because this indicator is usually observed later in the course of renal disease than the changes in creatinine or free water clearance, the fractional excretion of sodium is less beneficial for predicting imminent renal failure. [131] [132]
Free water clearance is a measure of the kidney's ability to dilute
or concentrate urine as necessary to maintain homeostasis. It is determined by the
following equation:
CH2
O
= UV − (UOsm
× UV/POsm
)
UV is urine volume, UOsm
is urinary osmolality, and POsm
is
plasma osmolality.
After an initial retrospective analysis, Jones and Weil[133] prospectively assessed the sensitivity of free water clearance and concluded that serial measurements predicted the patients who would experience progressive renal dysfunction.[109] In a prospective study, however, Rosenberg and coworkers[106] found no significant correlation between renal function and free water clearance in 69 patients with trauma or sepsis. After studying 38 patients in an intensive care unit, Baek and associates [134] concluded that the diagnosis of ARF was evident 1 to 3 days sooner with free water clearance than with other clinical and laboratory diagnostic criteria (creatinine clearance was not measured). These investigators recommended that sequential measurements of free water clearance could be used as an early indicator of pending ARF. A free water clearance rate of -15 to +15 mL/hr is a sign of incipient renal dysfunction. The normal range for free water clearance is -25 to -20 mL/hr.
After retrospectively reviewing the records of 675 trauma victims, Shin and colleagues[135] concluded that free water clearance alone was not sufficient for the early detection of renal dysfunction. They suggested that the combination of free water clearance greater than 20 mL/hr and creatinine clearance less than 25 mL/min predicted a high-risk group. When they prospectively monitored free water clearance in patients after surgery,[105] they concluded that this measure was not as sensitive as creatinine clearance for predicting ARF in trauma patients. The same substances and conditions that decrease the usefulness of urine osmolality for indicating renal failure can similarly decrease the usefulness of free water clearance (see Table 37-4 ).[136]
Creatinine clearance measures the ability of the glomeruli to
filter creatinine from the plasma and approximates the rate of glomerular filtration.
Measurements of creatinine clearance are most useful to quantify renal reserve.
Creatinine clearance often is estimated in the stable patient by use of the following
equation:
GRF = (1402Age) wt/72 × Serum creatinine level
The weight (wt) is weight in kilograms. However, this estimate is subject to the
same limitations as the measurement of serum creatinine. Precise measurements of
creatinine clearance require collection of timed urine samples using the following
formula:
GFR = UV/P
U is the urinary concentration of creatinine (mg/dL), V is the volume of urine (mL/min),
and P is the plasma concentration (mg/dL).
When Shin and associates[135] retrospectively reviewed the records of 26 patients who had acute tubular necrosis, they concluded that the diagnosis was likely to be made in patients who had a creatinine clearance of less than 25 mL/min and a free water clearance of greater than 15 mL/hr. In a follow-up study of 40 trauma patients who underwent surgery, Shin and colleagues [105] concluded that creatinine clearance of less than 25 mL/min alone permitted the early detection of renal dysfunction in patients with the potential for development of renal failure and that free water clearance was not as sensitive.
The major limitation in the determination of creatinine clearance is the necessity of collecting urine accurately. Although reasonable correlation between 2-hour and 24-hour creatinine clearance (R = 0.85) has been reported for intensive care patients[137] [138] ( Fig. 37-8 ), it remains true that the longer the urine collection period, the more accurate is the calculation of creatinine clearance. Changing hydration of the patient invalidates short-term determinations of the GFR, as does failure to record urine volume accurately. The error in calculation of creatinine clearance can vary from 10% to 27%, depending on the accuracy of urine collection,[139] body weight, surface area, and normal day-to-day variations. Another significant drawback is that for the patient with ARF, a test that requires 24-hour urine collection is often impractical. Nevertheless, it appears that creatinine clearance is the most efficient test available to assess glomerular filtration.
|
|
|
|
|
|
|
|
|
|
|
|
|