|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The most commonly used display mode is PCO2 versus time. Traditionally, several phases are distinguished in the capnograph trace. During inspiration and the first portion of expiration during which dead space gas is exhaled, there is no CO2 (phase I). As expiration continues, a short phase of the capnogram is recognized (phase II), with a rapid upstroke toward the alveolar plateau, representing the rising front of CO2 ( Fig. 36-17A ). The boundary of this front can be smeared by a variety of causes, but most notably by uneven mixing of the alveolar CO2 bolus in the airways. Phase III, also called the alveolar plateau, represents the constant or slowly upsloping part of the capnogram.
In the time capnogram, the alveolar plateau lasts for the greater part of the trace (see Fig. 36-17 ), although the expiratory flow is highest at the beginning of the expiration and tapers off in exponential fashion during the last third of expiratory time. Most of the exhaled volume of a passive expiration therefore exits the trachea (and the
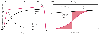 Figure 36-17
Time and volume capnographs. A,
Expired PCO2
versus time (i.e., standard
time capnogram). The waveform is conventionally subdivided into phases. During
phase I, exhaled gas from the large airways has a PCO2
= 0. Phase II is the transition between airway and alveolar gas. Phase III (i.e.,
alveolar plateau) is normally flat, but in the presence of V̇A/
Figure 36-17
Time and volume capnographs. A,
Expired PCO2
versus time (i.e., standard
time capnogram). The waveform is conventionally subdivided into phases. During
phase I, exhaled gas from the large airways has a PCO2
= 0. Phase II is the transition between airway and alveolar gas. Phase III (i.e.,
alveolar plateau) is normally flat, but in the presence of V̇A/![]() mismatching, it has a positive slope. The down slope of the capnogram at the onset
of inspiration is usually referred to as phase IV, but there is sometimes a terminal
increase in the slope associated with the onset of airway closure (dashed
line labeled IV'). This corresponds to the terminal upstroke seen in
single inert gas washout curves, referred to in that setting as phase IV.[322]
The PCO2
value at the end of exhalation
is referred to as the end-tidal PCO2
(PETCO2
).
Also shown are the exhaled gas flow rate and volume. B,
Volume capnogram. In this form of the capnogram, exhaled PCO2
is plotted against exhaled volume. Mixed expired PCO2
can be measured for each breath as the area under the capnogram. Total physiologic
dead space (VDS PHYS) can there fore be measured
using arterial PCO2
and Equation 12 (Bohr
equation, assuming PACO2
= PACO2
).
Line AC is drawn tangent to the terminal portion of the alveolar plateau. Vertical
line BE is constructed such that the two shaded areas (EDG and BCG) are equal in
area. FE therefore represents anatomic dead space (VDS ANAT),
[323]
which includes the volume in the trachea and
large airways and any volume within a breathing circuit in which exhaled gas is rebreathed,
such as the endotracheal tube, passive humidification device, or Y-piece. Alveolar
dead space (VDS ALV)[323]
can therefore be calculated as the difference between VDS PHYS
and VDS ANAT.[324]
Because the area of trapezoid BCDE is equal to the volume of CO2
exhaled
per breath, the mean (or average) alveolar PCO2
is the value at the midpoint of segment BC (point P).[324]
mismatching, it has a positive slope. The down slope of the capnogram at the onset
of inspiration is usually referred to as phase IV, but there is sometimes a terminal
increase in the slope associated with the onset of airway closure (dashed
line labeled IV'). This corresponds to the terminal upstroke seen in
single inert gas washout curves, referred to in that setting as phase IV.[322]
The PCO2
value at the end of exhalation
is referred to as the end-tidal PCO2
(PETCO2
).
Also shown are the exhaled gas flow rate and volume. B,
Volume capnogram. In this form of the capnogram, exhaled PCO2
is plotted against exhaled volume. Mixed expired PCO2
can be measured for each breath as the area under the capnogram. Total physiologic
dead space (VDS PHYS) can there fore be measured
using arterial PCO2
and Equation 12 (Bohr
equation, assuming PACO2
= PACO2
).
Line AC is drawn tangent to the terminal portion of the alveolar plateau. Vertical
line BE is constructed such that the two shaded areas (EDG and BCG) are equal in
area. FE therefore represents anatomic dead space (VDS ANAT),
[323]
which includes the volume in the trachea and
large airways and any volume within a breathing circuit in which exhaled gas is rebreathed,
such as the endotracheal tube, passive humidification device, or Y-piece. Alveolar
dead space (VDS ALV)[323]
can therefore be calculated as the difference between VDS PHYS
and VDS ANAT.[324]
Because the area of trapezoid BCDE is equal to the volume of CO2
exhaled
per breath, the mean (or average) alveolar PCO2
is the value at the midpoint of segment BC (point P).[324]
Why is the alveolar plateau upsloping in patients with diffuse
airways obstruction? Several effects are responsible. A perfect lung would have
perfect matching of ventilation and perfusion (V̇A/![]() = 1). However, diffuse airways obstruction is a nonuniform process
in which there are gas exchange units with a spectrum of V̇A/
= 1). However, diffuse airways obstruction is a nonuniform process
in which there are gas exchange units with a spectrum of V̇A/![]() ratios. Units with high V̇A/
ratios. Units with high V̇A/![]() ratios are
overventilated in relation to perfusion; alveolar PCO2
in such units is low. Gas exchange units with low V̇A/
ratios are
overventilated in relation to perfusion; alveolar PCO2
in such units is low. Gas exchange units with low V̇A/![]() ratios are underventilated in relation to perfusion and have high local alveolar
PCO2
. During exhalation, these gas exchange
units empty at different rates; units that are unobstructed (normal or high V̇A
/
ratios are underventilated in relation to perfusion and have high local alveolar
PCO2
. During exhalation, these gas exchange
units empty at different rates; units that are unobstructed (normal or high V̇A
/![]() ) empty quickly, followed by obstructed units. Gas exhaled early in the breath
tends to have a lower PCO2
than gas exhaled
later.
) empty quickly, followed by obstructed units. Gas exhaled early in the breath
tends to have a lower PCO2
than gas exhaled
later.
Another reason for the progressive rise in CO2 concentration is that during the exhalation, CO2 excretion from pulmonary capillaries into the alveoli continues at a nearly constant molar rate. CO2 molecules entering a lung volume
 Figure 36-18
Examples of capnograph waves. A,
Normal spontaneous breathing. B, Normal mechanical
ventilation. C, Prolonged exhalation during spontaneous
breathing. As CO2
diffuses from the mixed venous blood into the alveoli,
its concentration progressively rises (see Fig.
36-19
). D, Increased slope of phase III
in a mechanically ventilated patient with emphysema. E,
Added dead space during spontaneous ventilation. F,
Dual plateau (i.e. tails-up pattern) caused by a leak in the sample line.[325]
The alveolar plateau is artifactually low because of dilution of exhaled gas with
air leaking inward. During each mechanical breath, the leak is reduced because of
higher pressure within the airway and tubing, explaining the rise in the CO2
concentration at the end of the alveolar plateau. This pattern is not seen during
spontaneous ventilation because the required increase in airway pressure is absent.
G, Exhausted CO2
absorbent produces an
inhaled CO2
concentration greater than zero. H,
Double peak for a patient with a single lung transplant. The first peak represents
CO2
from the transplanted (normal) lung. CO2
exhalation from
the remaining (obstructed) lung is delayed, producing the second peak. I,
Inspiratory valve stuck open during spontaneous breathing. Some backflow into the
inspired limb of the circuit causes a rise in the level of inspired CO2
J, Inspiratory valve stuck open during mechanical
ventilation. The "slurred" downslope during inspiration represents a small amount
of inspired CO2
in the inspired limb of the circuit. K
and L, Expiratory valve stuck open during spontaneous
breathing or mechanical ventilation. Inhalation of exhaled gas causes an increase
in inspired CO2
. M, Cardiogenic oscillations,
when seen, usually occur with sidestream capnographs for spontaneously breathing
patients at the end of each exhalation. Cardiac action causes to-and-fro movement
of the interface between exhaled and fresh gas. The CO2
concentration
in gas entering the sampling line therefore alternates between high and low values.
N, Electrical noise resulting from a malfunctioning
component. The seemingly random nature of the signal perturbations (about three
per second) implies a nonbiologic cause.
Figure 36-18
Examples of capnograph waves. A,
Normal spontaneous breathing. B, Normal mechanical
ventilation. C, Prolonged exhalation during spontaneous
breathing. As CO2
diffuses from the mixed venous blood into the alveoli,
its concentration progressively rises (see Fig.
36-19
). D, Increased slope of phase III
in a mechanically ventilated patient with emphysema. E,
Added dead space during spontaneous ventilation. F,
Dual plateau (i.e. tails-up pattern) caused by a leak in the sample line.[325]
The alveolar plateau is artifactually low because of dilution of exhaled gas with
air leaking inward. During each mechanical breath, the leak is reduced because of
higher pressure within the airway and tubing, explaining the rise in the CO2
concentration at the end of the alveolar plateau. This pattern is not seen during
spontaneous ventilation because the required increase in airway pressure is absent.
G, Exhausted CO2
absorbent produces an
inhaled CO2
concentration greater than zero. H,
Double peak for a patient with a single lung transplant. The first peak represents
CO2
from the transplanted (normal) lung. CO2
exhalation from
the remaining (obstructed) lung is delayed, producing the second peak. I,
Inspiratory valve stuck open during spontaneous breathing. Some backflow into the
inspired limb of the circuit causes a rise in the level of inspired CO2
J, Inspiratory valve stuck open during mechanical
ventilation. The "slurred" downslope during inspiration represents a small amount
of inspired CO2
in the inspired limb of the circuit. K
and L, Expiratory valve stuck open during spontaneous
breathing or mechanical ventilation. Inhalation of exhaled gas causes an increase
in inspired CO2
. M, Cardiogenic oscillations,
when seen, usually occur with sidestream capnographs for spontaneously breathing
patients at the end of each exhalation. Cardiac action causes to-and-fro movement
of the interface between exhaled and fresh gas. The CO2
concentration
in gas entering the sampling line therefore alternates between high and low values.
N, Electrical noise resulting from a malfunctioning
component. The seemingly random nature of the signal perturbations (about three
per second) implies a nonbiologic cause.
PETCO2 can exceed arterial PCO2 .[174] [175] [176] [177] Other than calibration error, the most common cause is slow respiratory rate or high mixed venous PCO2 . In these situations the cyclic variations in alveolar PCO2 (which oscillates between arterial and mixed venous values) may be relatively large, causing the PETCO2 value to rise above the arterial value.
When patients are anesthetized in the lateral position, uneven ventilation of the dependent lung and lesser perfusion of the nondependent lung increase the range of alveolar gas disparity and the upslope of the alveolar plateau. This can be verified by alternating sampling sites between the two lungs when ventilating a patient in the lateral position with separate endobronchial tubes.
Sometimes, when expiration is prolonged and progresses to a lung volume below closing capacity, expired CO2 concentration may rise sharply at the end of the alveolar plateau, in a fashion analogous to that of N2 concentration after washout with 100% O2 .[178] One possible reason for this is that lung units subtended by airways predisposed to closure, at least in the spontaneously breathing patient, may contain alveolar gas with lower PaCO2 . Closure of these airways would therefore allow a greater proportion of CO2 -rich gas to reach the upper airway, producing the sharp upswing in CO2 concentration. An alternative hypothesis is that during the course of expiration, well-ventilated lung units have a progressive, nonlinear, upsloping increase in PCO2 , whereas that of poorly ventilated (closure-prone) units increases in a linear fashion. When airway closure occurs, the rate of rise in well-ventilated units predominates, and the slope of the capnogram abruptly increases.[179] Using the terminology of the single-breath washout curve, this portion of the capnogram is occasionally referred to as phase IV.
The last segment of a capnograph is represented by the beginning of inspiration, when the CO2 concentration rapidly decreases toward the inspired value. The capnogram value during inspiration represents the concentration of CO2 in the inspirate and depends mostly on the breathing circuit used and inspiratory flow and fresh gas flow values. Rebreathing of dead space volume is often the cause of an inspired level above baseline (see Fig. 36-18E ); this can be promptly corrected by removing the dead space. Exhaustion of the CO2 absorber may also result in an elevated value of inspired PCO2 (see Fig. 36-18G ). The sampling flow rate of sidestream monitors must be taken into account when interpreting many features of the capnogram profile. Usually, sampling flow rates vary, and they can be set between 50 and 400 mL/min, with an average optimal value for an adult patient of about 200 mL/min. As soon as the respiratory gas flows decrease below the sidestream gas sampling rate, the characteristics of the sampling vary; instead of sampling a portion of the mainstream flow, the equipment contributes significantly to bulk flow in and out of the respiratory circuit. This is of great importance in the pediatric setting, during low-flow anesthesia, and when interpreting capnograms obtained during very low respiratory rates. For example, during a prolonged expiration or an end-expiratory pause, while the gas flow exiting the trachea approaches zero, the sampling of the monitor may aspirate gas alternately from the trachea and the inspiratory limb.
A profile that illustrates this effect is shown in Figure 36-18M ; it is called cardiogenic oscillation, referring to the cause of the alteration. Synchronous changes in pulmonary blood flow during slow expiration and mechanical agitation of different lung regions induced by cardiac activity and
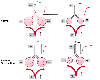 Figure 36-19
Mechanisms of airways obstruction producing an upsloping
phase III capnogram. In a normal, healthy person (upper panel), there is a narrow
range of V̇A/
Figure 36-19
Mechanisms of airways obstruction producing an upsloping
phase III capnogram. In a normal, healthy person (upper panel), there is a narrow
range of V̇A/![]() ratios with values close
to 1. Gas exchange units therefore have similar PCO2
and tend to empty synchronously, and the expired PCO2
remains relatively constant. During the course of exhalation, the alveolar PCO2
slowly rises as CO2
continuously diffuses from the blood. This causes
a slight increase in PCO2
toward the end
of expiration, and this increase can be pronounced if the exhalation is prolonged
(see Fig. 36-18C
). In a
patient with diffuse airways obstruction (lower panel), the airway pathology is heterogeneous,
with gas exchange units having a wide range of V̇A/
ratios with values close
to 1. Gas exchange units therefore have similar PCO2
and tend to empty synchronously, and the expired PCO2
remains relatively constant. During the course of exhalation, the alveolar PCO2
slowly rises as CO2
continuously diffuses from the blood. This causes
a slight increase in PCO2
toward the end
of expiration, and this increase can be pronounced if the exhalation is prolonged
(see Fig. 36-18C
). In a
patient with diffuse airways obstruction (lower panel), the airway pathology is heterogeneous,
with gas exchange units having a wide range of V̇A/![]() ratios. Well-ventilated gas exchange units, with gas containing a lower PCO2
,
empty first; poorly ventilated units, with a higher PCO2
,
empty last. In addition to the continuous rise in PCO2
mentioned previously, there is a progressive increase caused by asynchronous exhalation.
ratios. Well-ventilated gas exchange units, with gas containing a lower PCO2
,
empty first; poorly ventilated units, with a higher PCO2
,
empty last. In addition to the continuous rise in PCO2
mentioned previously, there is a progressive increase caused by asynchronous exhalation.
Inspection of the whole curve rather than measurement only of peak expired and peak inhalation values, which may be reported digitally, improves the scope and interpretation of the trace. When a capnographic trace is displayed such that all characteristics of the various stages of the waveform are visible on a rapidly scrolling oscilloscope or on fast-moving paper, it is possible to recognize several features that may be of diagnostic value. When capnograms are plotted on slow-moving paper or trends of only inspired and PETCO2 values are produced, it is still possible to recognize important clinical information regarding rising or falling concentrations of inspired and expired CO2 . Examples of capnographic waveforms are shown in Figure 36-18 , and additional waveforms and didactic material on capnography can be found on the Internet (www.capnography.com).
Normal end-expiratory CO2 partial pressure ranges between 35 and 45 mm Hg. An increase above this level (i.e., hypercapnia) must be interpreted in the light of additional information, because it depends on a variety of factors:
If ventilation is controlled, inadequate mechanical ventilation must be the first interpretation of increased end-tidal CO2 . If an increasing trend in end-tidal CO2 is observed while total ventilation remains constant, it is essential to verify the patient's temperature to exclude the diagnosis of hyperpyrexia. Other frequently observed causes of transient increases in end-tidal CO2 are release of tourniquets with reperfusion of ischemic areas, release of aortic clamps, intravenous administration of bicarbonate
 Figure 36-20
The effect of sodium bicarbonate administration on end-tidal
PCO2
. A continuous tracing of end-tidal
PCO2
is shown as a function of time.
Intravenous administration of 50 mEq followed by 30 mEq of NaHCO3
results
in an abrupt increase in expired CO2
because of neutralization of bicarbonate
by hydrogen ions.
Figure 36-20
The effect of sodium bicarbonate administration on end-tidal
PCO2
. A continuous tracing of end-tidal
PCO2
is shown as a function of time.
Intravenous administration of 50 mEq followed by 30 mEq of NaHCO3
results
in an abrupt increase in expired CO2
because of neutralization of bicarbonate
by hydrogen ions.
Abnormally low end-tidal values (<35 mm Hg) most often reflect hyperventilation but may also be caused by increased dead space with normal PaCO2 . For example, alveolar gas emanating from a lung region with no blood flow (and therefore no local CO2 transfer) dilutes exhaled gas and decreases PETCO2 relative to PaCO2 . Hypocapnia may also be artifactually induced by high sampling rate of the sidestream monitor in the face of an elevated fresh gas flow rate. Low PETCO2 may reflect decreased CO2 (PETCO2 during cardiac arrest may equal zero). The most common causes of an acute drop in PETCO2 are a decrease in cardiac output, regional hypoperfusion of the lung due to pulmonary embolism, and airway disconnection or occlusion.
Similarly, areas of lung that are perfused but unventilated (e.g., in atelectasis) because of shunting of mixed venous blood have high regional PaCO2 and high arterial minus end-tidal CO2 (PaCO2 - PETCO2 ), which has been used as a criterion for optimizing PEEP in ventilated patients.[180] Increasing levels of PEEP would be expected to resolve atelectasis and decrease PaCO2 - PETCO2 in these areas. In normal areas, overdistension impairs perfusion and therefore increases regional PaCO2 - PETCO2 . Optimal PEEP can be defined as when a balance is reached.
Irregularities in the alveolar plateau are often observed when mechanical factors acutely alter the pattern of alveolar emptying, such as when the arm of a surgeon compresses the chest in the middle of expiration. A cleft or a dip on the capnogram may indicate a spontaneous breath of small tidal volume, able to move just a small bolus of inspired gas past the sampling site. This often has been interpreted as indicating activation of respiratory centers by a patient recovering from anesthesia or activation of respiration induced by sudden increasing stimulation in the surgical field. It may also indicate inadequate inspiratory power during the switch from mechanical to spontaneous ventilation and can be the first sign of the need for reversal of neuromuscular blockade. However, it may indicate just the opposite, the need for increased total ventilation to induce apnea in the patient. The dip in the plateau of the capnogram must be interpreted with care, depending on the surgical stage, the drug exposure history of the patient, and the anesthetic plan.
The main use of the capnographic signal in anesthesia is the immediate verification of tracheal intubation beyond doubt by the immediate and continuous presence of metabolic CO2 in the expired gas. Esophageal intubation may produce one or a few breaths containing CO2 during expiration, but the concentration of this gas in exhalations from the stomach cavity rapidly decreases to zero. In this fashion, the continuous appearance of expired CO2 is rapidly being adopted as a criterion for correct tracheal intubation. Another major use of this signal has been to determine the correct ventilatory needs (e.g., minute ventilation) during controlled ventilation. This use is easily extended to continuous monitoring of spontaneous ventilation and to monitoring of appropriate ventilation during titration of anesthetic agents that depress ventilation. In partial rebreathing circuits and in low-flow anesthesia, capnometry facilitates the adjustment of fresh gas flow, which is a major determinant of CO2 levels insofar as it may increase minute ventilation. The shape of a partial rebreathing capnogram may vary greatly, depending on the ventilatory frequency and tidal volume. In all of these modes of use, the sensing head of the capnograph or the site of gas sampling is best kept close to the tracheal tube, connected with the minimal dead space assembly. In this mode, for instance, it is easy to reveal disconnection of the breathing system or inappropriate functioning of mechanical ventilators. Partial rebreathing circuit systems include a CO2 absorber, whose exhaustion is manifested by hypercarbia.
Clinical signs of hypercarbia may be only slowly recognized, whereas an increase in inspired CO2 is immediately observable with a capnograph. Rapid alterations in end-tidal CO2 can be recognized within a single exhalation. In a different setting, capnography has been used on the exhaust side of a cardiopulmonary bypass oxygenator to track CO2 excretion at different body temperatures.
If exhaled volume and PCO2 are measured simultaneously, exhaled PCO2 can be plotted against exhaled gas volume (see Fig. 36-17 ). Volume capnography has several advantages over time capnography. First, the CO2 signal can be integrated to obtain the volume of CO2 exhaled per breath, obtaining an on-line, breath-by-breath measurement of CO2 . Second, significant changes in the morphology of the expired waveform can be detected in the volume capnogram (e.g., resulting from PEEP) but not in the traditional time capnogram.[181] Third, dead space can be partitioned into components of interest. Total physiologic dead space (VD PHYS) can therefore be measured using arterial PCO2 and Equation 12 (Bohr equation, assuming PACO2 = PaCO2 ). Anatomic dead space (VD ANAT), including gas volume within a breathing circuit in which exhaled gas is rebreathed, such as the endotracheal tube, passive humidification device, or Y-piece, can be calculated
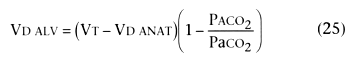
Normally, alveolar PCO2 can be estimated using PETCO2 obtained from the time capnogram as a measure of PACO2 . However, when the alveolar plateau has a significant slope, PETCO2 can exceed PaCO2 ,[174] [175] [176] [177] and the average alveolar PCO2 (see Fig. 36-17 ) obtained from the volume capnogram may be a more appropriate measure. [181] Commercial monitors incorporating volume capnometry are available.
|
|
|
|
|
|
|
|
|
|
|
|
|