|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indirect assessment of CVP through physical examination of the neck veins is a fundamental aspect of cardiovascular assessment, but one that has many shortcomings. One real anatomic limitation to the physical assessment of jugular venous pulsations results from the presence of competent venous valves that exist in the internal jugular veins of most individuals. These valves are located approximately 0.5 to 2.0 cm above the junction of the internal jugular and subclavian veins bilaterally and withstand backpressures of 50 to 100 cm H2 O.[170] [171] [172] [173] When CVP is increased, these valves are either incompetent or absent, particularly in patients with chronic severe tricuspid valve regurgitation.[173] In addition, the jugular veins may be impossible to identify in 20% of patients, and the bedside diagnosis of low, normal, or high CVP is often inaccurate, particularly in critically ill patients.[174] [175] [176] These general problems are compounded in the perioperative period, when visualization of the neck veins is further obscured and acute, large changes in CVP occur in many patients. As a result, direct measurement of CVP is performed frequently in hemodynamically unstable patients and those undergoing major operations.
Cannulation of a large central vein is the standard clinical method for monitoring CVP and is also performed for a number of additional therapeutic interventions, such as providing secure vascular access for the administration of vasoactive drugs or to initiate rapid fluid resuscitation. Frequently, the central venous location is the only site available for intravenous access of any kind. Patients at risk for venous air emboli may have central venous catheters placed for aspiration of entrained air. Central venous access is required to initiate transvenous cardiac pacing, temporary hemodialysis, or pulmonary artery catheterization for more comprehensive cardiac monitoring ( Table 32-5 ).
In certain patients, such as those with ischemic heart disease, the cardiovascular consequences of pain or anxiety
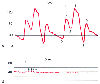 Figure 32-20
Unusual arterial pressure waveforms. A,
Intra-aortic balloon counterpulsation with a 1:2 balloon-assist ratio produces a
characteristic change in the arterial pressure waveform. Four cardiac cycles are
shown, two with balloon assistance and two without. 0, Unassisted end-diastolic
pressure; 1, unassisted systolic pressure; 2, dicrotic notch; 3, assisted or augmented
diastolic pressure; 4, end-diastolic or presystolic dip; 5, assisted systolic pressure.
Effective afterload reduction by the intra-aortic balloon is demonstrated by the
presystolic dip pressure (4) lower than the unassisted end-diastolic pressure (0)
and the assisted systolic pressure peak (5) lower than the unassisted systolic pressure
peak (1). B, Arterial pressure waveform during cardiopulmonary
bypass. Small phasic pressure variations (arrows)
result from the mechanical action of the bypass roller pump. The bypass pump flow
rate may be estimated by measuring these pulsations. Nineteen pulsations are recorded
in a 3-second time interval. In this case, the pump configured with ⅜-inch
tubing has an effective stroke volume of 27 mL. The pump flow rate may be calculated
as follows: (19 pulsations/3 seconds) × (1 pump revolution/2 pulsations)
× (27 mL/revolution) × (60 sec/min) = 5130 mL/min.
This calculated pump flow rate should equal the flow rate displayed on the pump
console (i.e., 5.2 L/min). (Redrawn from Mark JB: Atlas of Cardiovascular
Monitoring. New York, Churchill Livingstone, 1998, Figs. 20-3 and 19-8.)
Figure 32-20
Unusual arterial pressure waveforms. A,
Intra-aortic balloon counterpulsation with a 1:2 balloon-assist ratio produces a
characteristic change in the arterial pressure waveform. Four cardiac cycles are
shown, two with balloon assistance and two without. 0, Unassisted end-diastolic
pressure; 1, unassisted systolic pressure; 2, dicrotic notch; 3, assisted or augmented
diastolic pressure; 4, end-diastolic or presystolic dip; 5, assisted systolic pressure.
Effective afterload reduction by the intra-aortic balloon is demonstrated by the
presystolic dip pressure (4) lower than the unassisted end-diastolic pressure (0)
and the assisted systolic pressure peak (5) lower than the unassisted systolic pressure
peak (1). B, Arterial pressure waveform during cardiopulmonary
bypass. Small phasic pressure variations (arrows)
result from the mechanical action of the bypass roller pump. The bypass pump flow
rate may be estimated by measuring these pulsations. Nineteen pulsations are recorded
in a 3-second time interval. In this case, the pump configured with ⅜-inch
tubing has an effective stroke volume of 27 mL. The pump flow rate may be calculated
as follows: (19 pulsations/3 seconds) × (1 pump revolution/2 pulsations)
× (27 mL/revolution) × (60 sec/min) = 5130 mL/min.
This calculated pump flow rate should equal the flow rate displayed on the pump
console (i.e., 5.2 L/min). (Redrawn from Mark JB: Atlas of Cardiovascular
Monitoring. New York, Churchill Livingstone, 1998, Figs. 20-3 and 19-8.)
| Central venous pressure monitoring |
| Pulmonary artery catheterization and monitoring |
| Transvenous cardiac pacing |
| Temporary hemodialysis |
| Drug administration |
| Concentrated vasoactive drugs |
| Hyperalimentation |
| Chemotherapy |
| Drugs irritating to peripheral veins |
| Prolonged antibiotic therapy (e.g., endocarditis) |
| Rapid infusion of fluids (through large cannulas) |
| Trauma |
| Major surgery |
| Aspiration of air emboli |
| Inadequate peripheral intravenous access |
| Sampling site for repeated blood testing |
Many different techniques for internal jugular vein cannulation have been described, although the "central" approach described by Daily and colleagues is among the most popular and is described with minor modifications here.[181] Careful positioning will make the patient comfortable, improve identification of surface landmarks, and increase the likelihood of successful venipuncture. The patient is placed in the supine position with the head turned slightly to the left to expose the right side of the neck and keep the chin from interfering with the procedure. Pillows that cause the neck to be flexed should be removed, but forceful neck extension or extreme leftward rotation of the head should be avoided because this may alter cervical vascular anatomy, cause the internal jugular vein to overlie the carotid artery, and increase the risk of puncture of the carotid artery.[182]
Anatomic landmarks, including the sternal notch, clavicle, and sternocleidomastoid muscle, should be assessed before preparation and draping for the sterile procedure because these landmarks are better appreciated before they are covered by the sterile drape ( Fig. 32-21 ). The carotid artery should be palpated and its course determined; it lies lateral to the trachea, usually under the more medial sternal head of the sternocleidomastoid muscle. The internal jugular vein lies in the groove between the sternal and clavicular heads of the sternocleidomastoid muscle, lateral and slightly anterior to the carotid artery. In many patients, venous pulsations from the internal jugular vein are observed directly within this groove and further identify the approximate site for venipuncture.
The patient should be calm, sedated, receiving supplemental oxygen if necessary, and monitored with an ECG, blood pressure monitor, and pulse oximeter. Because of the frequency and serious morbidity of infectious complications from central venous catheterization, strict aseptic technique is required. Formal guidelines to reduce these complications have been published by the Centers for Disease Control and Prevention and provide prudent recommendations that should be followed during all but the most emergent catheterizations.[183] [184]
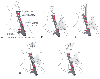 Figure 32-21
Technique for central venous cannulation of the right
internal jugular vein. A, Important surface landmarks
are identified. B, The course of the internal carotid
artery is palpated. C, The internal jugular vein
is punctured at the apex of the triangle formed by the two heads of the sternocleidomastoid
muscle, with the needle tip directed toward the ipsilateral nipple. D,
A guidewire is introduced through the thin-walled needle into the vein. E,
The central venous cannula is inserted over the guidewire while making sure that
the proximal end of the guidewire protrudes beyond the catheter and is controlled
by the operator.
Figure 32-21
Technique for central venous cannulation of the right
internal jugular vein. A, Important surface landmarks
are identified. B, The course of the internal carotid
artery is palpated. C, The internal jugular vein
is punctured at the apex of the triangle formed by the two heads of the sternocleidomastoid
muscle, with the needle tip directed toward the ipsilateral nipple. D,
A guidewire is introduced through the thin-walled needle into the vein. E,
The central venous cannula is inserted over the guidewire while making sure that
the proximal end of the guidewire protrudes beyond the catheter and is controlled
by the operator.
Good aseptic technique begins with hand washing before the procedure. A mask, cap, sterile gloves, and gown should always be used, even when placing the catheter in the operating room. The skin is cleansed widely from earlobe to clavicle to sternal notch with an appropriate antiseptic. Recent evidence suggests that 2% chlorhexidine may be preferred over the more widely used 10% povidone-iodine solution. [183] [185] A meta-analysis showed a 50% reduction in bloodstream infection when chlorhexidine was used for skin disinfection rather than povidone-iodine.[186] The aseptic preparation is completed with the application of a large sterile drape that provides maximal barrier precautions.
Under these sterile conditions, the relevant anatomy is again identified, particularly the course of the carotid artery in the neck. An assistant then places the patient in a slight head-down (Trendelenburg) position to increase the diameter of the jugular vein in the neck. This step is occasionally omitted in a hypervolemic, dyspneic patient. The intended venipuncture site is anesthetized by subcutaneous infiltration of a local anesthetic solution (typically 1% lidocaine) with a 25- or 26-gauge needle. The local anesthetic wheal should be generous enough to allow pain-free suturing of the catheter insertion point at the end of the procedure.
With the fingers of the left hand gently resting on the carotid artery pulse as a valuable anatomic landmark, venipuncture then proceeds with a 22-gauge, 1½-inch (3.8-cm) finger needle mounted on a 5-mL syringe (see Fig. 32-21 ). The needle is inserted at the apex of the triangle formed by the two heads of the sternocleidomastoid muscle, at an angle of approximately 30 degrees from the plane of the skin, and directed at the ipsilateral nipple. Gentle aspiration will identify the jugular vein when dark venous blood enters the syringe. Even though use of the small finder needle is an extra step in this procedure, it presumably increases the margin of safety[187] because unintentional puncture of the carotid artery with this small needle is less likely to result in significant bleeding and hematoma formation. Although blood may have been aspirated earlier during injection of the local anesthetic, the color of the blood may not be appreciated when it mixes with the local anesthetic solution, thereby making it difficult to distinguish venous from arterial blood. Thus, it is important that the finder needle be attached to an empty syringe during this step.
If blood is not aspirated as the finder needle is advanced and then withdrawn, additional needle passes may locate the internal jugular vein by fanning laterally in a small arc from the point where the needle enters the skin. As long as the carotid artery remains palpable medially, exploring in an orderly fashion with the finder needle directed slightly more laterally will often identify the vein with little risk of carotid artery puncture. If the vein is not located after several needle passes, the finder needle is withdrawn completely and checked for patency,
After the internal jugular vein is located with the finder needle, the needle is gently withdrawn while the skin and surface anatomy remain fixed by the left hand. The vein is then punctured with an 18-gauge, 2½-inch (6.4 cm) thin-walled needle attached to a 5-mL syringe directed along the same track used by the finder needle while keeping in mind the location and depth of the identified internal jugular vein. It is common for the lumen of the jugular vein to be compressed as this larger thin-walled needle is advanced, and as a result, the needle pierces both front and back walls almost simultaneously.[188] [189] Accordingly, the thin-walled needle should be inserted only slightly beyond the expected depth of the vein and then slowly withdrawn while maintaining gentle aspiration on the syringe. Frequently, venous entry of the needle is recognized during needle withdrawal by the sudden return of free-flowing venous blood.
Once successful venipuncture is confirmed by free aspiration of dark venous blood into the 5-mL syringe, the hub of the thin-walled needle is fixed with the fingers of the left hand, the syringe is detached, and venous blood should be observed to drip from the needle hub. A guidewire (generally 0.032 or 0.035 inch) is inserted through the needle by using either the J-shaped tip or the soft, flexible straight end. The wire should advance easily into the vein with little resistance. The ECG is monitored continuously to detect arrhythmias, which are common if the wire tip contacts the walls of the right atrium or ventricle.[190] From this point in the procedure, it is critical that the clinician maintain control over the guidewire and pay attention to its depth of insertion and continued sterility.
The puncture site is enlarged with a No. 11 scalpel blade to the size required for the intended catheter. A firm, tapered-tip vessel dilator may be inserted to dilate the subcutaneous tissues around the guidewire and allow a larger catheter to pass more smoothly. (When an introducer sheath is to be used for internal jugular vein cannulation, the dilator and sheath form one unit, and this separate dilation step is usually omitted.) The vessel dilator is removed, and the central venous catheter is inserted over the guidewire while traction on the skin is maintained. Again, the clinician must be attentive to the guidewire and ensure that a sufficient length protrudes from the catheter hub so that the wire may be extracted easily. The catheter is inserted to an appropriate depth that will place the tip in the superior vena cava, above its junction with the right atrium. This depth is typically 15 to 18 cm if the catheter is placed with the technique described.
Finally, the guidewire is withdrawn, the catheter is attached by a Luer-Lock connector to the monitoring or infusion tubing and sutured in place, and sterile gauze or transparent dressing is applied. Antibiotic ointment should not be applied to the insertion site because of the possibility of increasing the risk of catheter colonization with multidrug-resistant bacteria or Candida. [183] [184] [191]
Central venous catheters placed in the operating room are generally used for the duration of the surgical procedure without first confirming the location of the catheter tip radiographically. Before monitoring or infusion commences, aspiration of blood should confirm the intravenous location of each lumen of a multilumen catheter and remove any residual air from the catheter-tubing system. After surgery, however, the position of the catheter tip must always be confirmed radiographically. Catheter tips located within the heart or below the pericardial reflection on the superior vena cava increase the risk of cardiac perforation and fatal cardiac tamponade. Ideally, the catheter tip should lie within the superior vena cava, parallel to the vessel walls, and be positioned below the inferior border of the clavicles and above the level of the third rib, the T4 to T5 interspace, the azygos vein, the tracheal carina, or the takeoff of the right main stem bronchus.[192] [193] Using fresh human cadavers, Albrecht and colleagues recently confirmed that the tracheal carina was always above the pericardial reflection on the superior vena cava, thus suggesting that catheter tips should always be located superior to this radiographic landmark.[194]
Since its introduction into clinical practice in the late 1960s, [181] [195] [196] percutaneous venipuncture of the right internal jugular vein has been the method preferred by anesthesiologists for central venous cannulation.[197] Reasons for this preference include the consistent, predictable anatomic location of the internal jugular vein; readily identified, palpable surface landmarks; and a short, straight course to the superior vena cava, which facilitates right heart catheterization. An internal jugular vein catheter is more accessible intraoperatively to the anesthesiologist, who is most often working at the patient's head, and perhaps of greatest importance, vascular cannulation is highly successful, generally reported in the 90% to 99% range.[197] [198] [199]
Numerous techniques have been described for percutaneous internal jugular vein cannulation.[200] [201] [202] Although it is unlikely that one single method will lead to the greatest success rate in the hands of all clinicians, it appears that certain techniques may result in a higher incidence of unintentional carotid artery puncture.[201] It seems prudent, therefore, to determine the location of the carotid pulse before attempted jugular vein cannulation and to continue to palpate the pulse gently during the initial steps of venipuncture while being careful to avoid excessive pressure that may compress the jugular vein. A right-handed operator easily does this, but continued palpation of the carotid artery during jugular venipuncture is awkward when a left-handed individual is performing the procedure. In this instance, it is best to evaluate the course of the carotid artery before, but not during the venipuncture.
Other adjunctive measures should be considered in an attempt to reduce complications during central venous cannulation, particularly the frequency of carotid arterial puncture during attempted internal jugular cannulation. Many individuals substitute a 2-inch, 18-gauge intravenous
Widespread adoption of guidewire-based vascular cannulation methods has undoubtedly increased the safety of central venous cannulation. These techniques, originally described by Seldinger,[206] allow initial vascular puncture with a smaller-gauge needle, followed by guidewire placement and subsequent vessel dilation and cannulation with a large-bore catheter.[207] This method has virtually replaced the "catheter through the needle" method, which requires vascular puncture with a needle larger than the catheter and results in more complications from cannulation site hemorrhage and shearing of the catheter if it is withdrawn inappropriately through the insertion needle. Some central venous catheter insertion kits contain a modified syringe that allows the guidewire to be placed directly through the syringe plunger without disconnecting the syringe from the thin-walled needle. However, when this technique is used, one of the most valuable signs of unintentional arterial puncture is virtually impossible to recognize—namely, the pulsatile return of bright red blood through the thin-walled needle. Failure to recognize this sign may lead to erroneous arterial placement of a central venous catheter.[208]
Central venous catheters come in a variety of lengths, gauges, compositions, and lumen numbers.[209] [210] These features vary according to the purpose of catheterization, whether for CVP monitoring or other therapeutic needs and whether intended for short- or long-term use. It is therefore critical that the physician choose the best catheter for any given application and thereby reduce risk to the patient caused by improper catheter selection. Although multilumen catheters are very popular because they allow simultaneous continuous pressure monitoring and fluid or drug infusion, their use has been associated with a greater risk of infection than has the use of single-lumen catheters.[184] Furthermore, multilumen catheters may have a greater propensity than single-lumen catheters to cause vascular perforation because more septations and stiffer plastic are required in the manufacturing process of a multilumen catheter.[209] A popular alternative method for multilumen central venous access involves the use of a sidearm introducer sheath attached to a series of stopcocks for multiple drug infusions, with a single-lumen catheter inserted through the hemostasis valve used for simultaneous continuous CVP monitoring. Although the use of these larger introducer sheaths is not free from complications, they allow rapid placement of a pacing wire or pulmonary artery catheter for more intensive monitoring without the need for additional central venipunctures or catheter exchanges over a guidewire.
In patients at risk for major intraoperative blood loss and hemodynamic instability, two central venous catheters are frequently inserted. Some physicians advocate double cannulation of the same central vein (usually the right internal jugular) with two catheters in close proximity. In this technique, a guidewire is introduced into the internal jugular vein by the standard method. Then, before catheter placement, a second jugular venipuncture is performed, approximately 1 to 2 cm cephalad or caudad, and a second guidewire is introduced into the vein. Placing appropriate catheters over each guidewire and securing them at the skin in normal fashion completes the procedure. Limited evidence suggests that the incidence of major complications with this double-cannulation technique is no greater than with single central venous catheterization, although arrhythmias may be more common because of unintentional intracardiac placement of the guidewires.[211] However, it is not clear whether this approach is safer than central venous cannulation in two separate central veins. Serious reported complications of the double-cannulation technique include facial vein avulsion, catheter entanglement, and catheter fracture. [211] [212] [213] Double central venous cannulation must be reserved for patients whose needs for venous access and hemodynamic monitoring cannot be met through single cannulation.
Selecting the best site for safe and effective central venous cannulation ultimately requires that the physician consider the purpose of catheterization (pressure monitoring versus drug or fluid infusion), the patient's underlying medical condition, the intended operation, and the skill and experience of the physician performing the procedure. In patients with severe bleeding diatheses, it is best to choose a puncture site where bleeding from the vein or adjacent artery is easily detected and controlled with local compression. In this instance, an external jugular approach would be preferred over infraclavicular subclavian catheterization. Patients with severe emphysema or other patients who would be severely compromised by pneumothorax would be better candidates for right internal jugular cannulation than subclavian cannulation because of the higher risk of pneumothorax with the latter approach. If transvenous cardiac pacing were required in an emergency situation, catheterization of the right internal jugular vein is recommended because it provides the most direct course to the right ventricle. Trauma patients with their necks immobilized in a hard cervical collar are best resuscitated with the use of femoral or subclavian cannulas; the latter may be placed even more safely if the risk of pneumothorax is obviated by previous placement of thoracostomy tubes. The physician must recognize that the length of catheter
To increase the success and decrease the complications of central venous cannulation, different ultrasound-guided techniques have been described for cannulating the internal jugular vein,[215] [216] [217] [218] subclavian vein,[219] [220] [221] [222] and femoral vein.[223] Some methods use devices incorporated into the sterile field that provide real-time, two-dimensional ultrasound images of the pertinent vascular anatomy, particularly the relationship between the target vein and its adjacent artery. Alternatively, when a diagnostic echocardiograph machine is already available in the operating room, a standard short-focus surface transducer can be used to image the vascular anatomy ( Fig. 32-22 ). Intraoperative transesophageal echocardiography has been advocated to guide central venous catheterization for congenital heart surgery.[224] Finally, Doppler-based nonimaging techniques provide a real-time, audible Doppler signal to guide venous cannulation based on distinguishing the continuous audible hum of the vein from the pulsatile signal of the artery.
In general, these ultrasound-guided techniques appear to have several advantages. Invariably, with ultrasound assistance, fewer needle passes are required for successful venous cannulation. In addition, most investigators have shown that ultrasound guidance reduces the time required for catheterization, increases overall success rates, and results in fewer complications.[225] [226] In one of the largest controlled trials, Denys and coworkers used dedicated two-dimensional real-time imaging to cannulate the internal jugular vein in more than 900 patients and found that
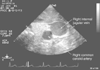 Figure 32-22
Transverse-plane ultrasound image showing the right internal
jugular vein and its typical anatomic position anterior and lateral to the right
common carotid artery.
Figure 32-22
Transverse-plane ultrasound image showing the right internal
jugular vein and its typical anatomic position anterior and lateral to the right
common carotid artery.
Most published studies support the use of ultrasound guidance during central venous cannulation, and a recent report from the Agency for Healthcare Research and Quality advocates more widespread adoption of this practice.[226] Nonetheless, many of these procedures are still performed with landmark-based techniques because of the relatively high success rate and low morbidity of this procedure when performed by experienced individuals, the additional cost and perceived inconvenience of acquiring and using an ultrasound device, and concern that reliance on ultrasound-guided catheterization will prevent trainees from acquiring adequate skills for landmark-based central venous cannulation.
Notwithstanding these concerns, ultrasound studies have disclosed several important anatomic and technical insights that should lead to improved central venous cannulation in all patients, regardless of the technique used. The large central veins are readily distinguished from their accompanying arteries by their lack of pulsatility, marked enlargement during a Valsalva maneuver, and easy compressibility with the ultrasound probe. This provides several lessons for landmark-based venipuncture. When the internal jugular vein is not located easily with the finder needle, the clinician can increase the venous target size markedly by asking the patient to perform a Valsalva maneuver. In contrast, jugular venipuncture will be much more difficult if the vein is compressed during overzealous attempts to palpate and localize the carotid artery. Ultrasound imaging has demonstrated that both the subclavian[219] and internal jugular[216] veins are actually compressed, before vessel entry, by the cannulating needle as it advances. This observation explains the common clinical finding that venipuncture is recognized as often during needle withdrawal as during needle advancement. Furthermore, ultrasonography demonstrates that in a significant number of patients, the internal jugular vein lies directly over the carotid artery,[227] [228] thereby increasing the risk of carotid artery puncture unless the needle is directed more laterally or lower in the neck where the internal jugular vein assumes a more lateral location.[218] Ultrasound-guided techniques may be particularly useful for increasing the success rate and decreasing complications when less experienced individuals and trainees perform central venous cannulation. [216] [219] Perhaps of greatest importance, ultrasound-guided central venous cannulation provides a method to salvage the procedure when landmark-based methods are unsuccessful, thus making its use particularly attractive in high-risk patients in whom the clinician anticipates difficulty with vascular access.[216] [218] [219]
Left internal jugular vein cannulation may be accomplished with a technique similar to the one described earlier for the right internal jugular vein, although several anatomic details make the left side less attractive than the right.
The subclavian vein is an important site for central venous cannulation and is particularly popular among surgeons and other physicians who place central venous catheters for emergency volume resuscitation and long-term intravenous therapy rather than for shorter-term monitoring purposes.[222] [231] [232] Advantages of subclavian venous cannulation include a lower risk of infection than with internal jugular or femoral sites,[183] [184] [233] [234] ease of insertion in trauma patients who may be immobilized in a cervical collar, and increased patient comfort, especially for long-term intravenous therapy such as hyperalimentation and chemotherapy.
The most common technique used for subclavian vein cannulation is the infraclavicular approach.[232] The patient is placed in a slight head-down position with the arms fully adducted, the head is turned slightly away from the side of venipuncture, and a small bedroll is placed between the shoulder blades to fully expose the infraclavicular area. The skin is punctured 2 to 3 cm caudad to the midpoint of the clavicle, far enough from its inferior edge to avoid downward angulation of the needle as it is inserted just beneath the posterior surface of the clavicle. The needle tip is directed toward the suprasternal notch, which may be constantly identified by the fingers of the operator's other hand. If the subclavian vein is not entered in the first pass, the needle may be withdrawn and a second pass attempted in a slightly more cephalad direction while ensuring that the needle continues to hug the undersurface of the clavicle as it is advanced. Once the subclavian vein is punctured, catheterization proceeds in a manner similar to that described for jugular vein catheterization.
Several important technical details must be followed to ensure successful subclavian vein cannulation and avoid complications, particularly pneumothorax. Venipuncture generally proceeds without first identifying the vein with a smaller-gauge finder needle because the depth of the subclavian vein is often beyond the reach of the standard 1½-inch, 22-gauge needle. A thin-walled needle is preferred over an 18-gauge catheter for venipuncture and guidewire placement because the 18-gauge catheter is easily kinked as it courses under the clavicle. Of greatest importance, however, the clinician should resist the temptation to make multiple needle thrusts if the subclavian vein is not punctured on the second or third attempt. It is clear that complications from this procedure, particularly the incidence of pneumothorax and subclavian artery puncture, are directly related to the number of attempts and are more common when venipuncture is unsuccessful.[222] [232] [234] Bilateral attempts at subclavian venipuncture should rarely be undertaken because of the potential serious morbidity of bilateral pneumothorax. Perhaps even more than in the case of internal jugular vein cannulation, the safety of subclavian venipuncture rests in the experience of the operator. In more practiced hands, the incidence of complications should be lower, with pneumothorax developing in less than 2% and arterial puncture in less than 5% of cases.[222] [232]
Both the right and left external jugular veins provide a safe alternative to internal jugular or subclavian vein cannulation. Because the external jugular veins are superficial, they allow central venous cannulation with essentially no risk of pneumothorax or unintended arterial puncture. In most instances, it is best to use an 18-gauge catheter rather than the thin-walled needle to introduce the guidewire because of the tortuous course of the external jugular vein and the frequent need to manipulate the guidewire repeatedly to direct it into the superior vena cava. A J-tipped guidewire should always be used because it may be advanced under the clavicle into the central circulation more successfully than a straight-tipped wire.[235] [236] When the guidewire does not advance as desired and appears to be moving peripherally into the subclavian vein, abducting the ipsilateral shoulder beyond 90 degrees before advancing the wire may facilitate central venous passage. Alternatively, the patient's ipsilateral arm is placed at the side, and an assistant applies mild caudad traction on the shoulder to straighten the course of the external jugular vein while the wire is advanced. Essentially the only factors that preclude use of the external jugular veins for CVP monitoring are an inability to visualize and cannulate the vessel in the neck and advance the catheter into the central circulation. Unfortunately, these problems occur in approximately 20% of patients, thus limiting more widespread application of this technique.[203] [237]
Femoral vein cannulation provides a useful site for CVP monitoring when the more common jugular and subclavian sites are not accessible, as in patients with burns, trauma, or surgical procedures that involve the head, neck, and upper part of the thorax or during cardiopulmonary resuscitation. Use of the femoral vein obviates many of the common complications of central venous catheterization, particularly pneumothorax and carotid or subclavian artery injury. Femoral venipuncture is performed below the inguinal ligament just medial to the palpated femoral arterial pulse in a manner similar to
In patients with extensive, severe burn injuries, the axillary region is often spared and provides a useful site for either arterial or venous pressure monitoring.[240] Standard 20-cm CVP catheters placed in the axillary veins, approximately 1 cm medial to the palpated axillary artery, will allow measurement of pressure from the superior vena cava. Even more distal pressures measured from peripheral veins in the hand and forearm may provide a reasonably accurate estimate of CVP in selected surgical patients.[241] Modified volumetric infusion pumps can measure in-line peripheral venous pressure without the need for additional transducers and monitoring equipment.[242] Although this method of measuring CVP incurs no risk beyond that associated with placing any standard peripheral intravenous catheter, it has not been widely validated and cannot replace central venous cannulation in most circumstances.
Peripherally inserted central venous catheters (PICCs) have become a popular alternative to centrally inserted catheters in patients requiring long-term intravenous therapy. Advantages of PICCs include bedside placement under local anesthesia, extremely low risk of major insertion-related complications, and safe placement by nonphysicians (i.e., registered nurses and physician assistants). This technique may be particularly cost-effective because it eliminates the need for a minor operative procedure in patients who require a Hickman or Broviac central venous catheter.[243] Venous access for a PICC is obtained through an antecubital vein, preferably the basilic vein, which is generally more successfully catheterized than the cephalic vein because the latter is more tortuous, particularly at the clavipectoral fascia. Early reports described only modest success positioning PICCs in an appropriate central location, as well as a considerable risk of venous thrombosis,[244] [245] [246] but improvements in catheter design and insertion technique now result in successful placement and few complications in most patients.[243] Most PICCs placed for long-term therapeutic indications (chemotherapy or parenteral nutrition) are very flexible, nonthrombogenic silicone catheters. Less commonly, a standard polyurethane 40-cm intravenous catheter is inserted peripherally and advanced to a central location for short-term infusion of vasoactive drugs or monitoring of CVP or PAP. When these standard long venous catheters are inserted from an antecubital vein, the catheter tip may advance into the heart as the arm is abducted, thereby increasing the risk of cardiac perforation or arrhythmias.[247] [248] Whenever a PICC line is in place, the clinician should exercise caution when placing any additional central venous catheters because of the risk of shearing the PICC line within the central venous circulation.
|
|
|
|
|
|
|
|
|
|
|
|
|