|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anatomically, many of the body's muscles exert their force on tendons that glide around bony tubercles or through tendonous retinacula. This enables the muscles to exert their actions around joints whether the joints are extended or flexed. Some nerves follow similar anatomic courses. Although excessive flexion or extension can produce predictable problems in patients' nerves and muscles through excessive pressure and stretch, other mechanisms can also lead to positioning injuries.
Dylewsky and McAlpine[7] described four axonal reactions to injury. Transient ischemic nerve blocks occur with no structural nerve damage and last only minutes. Neurapraxia recovery takes 4 to 6 weeks and results from demyelination of peripheral fibers of the nerve trunk. Axonotmesis describes a complete disruption of the axons within an intact nerve sheath; recovery depends on regeneration of the distal nerve at 1 mm per day, but complete recovery is unlikely. Neurotmesis involves complete nerve disruption; surgical repair can produce only partial functional recovery at best.
Five in vivo mechanisms for perioperative peripheral neuropathies are stretch, compression, generalized
Metabolic diseases play a role in perioperative neuropathies such as diabetes, and nutritional problems such as pernicious anemia and alcoholic neuritis are common in surgical patients. Arteriosclerosis, drug, or heavy metal exposure and infectious diseases such as polio also can cause neuropathies. Perioperative cigarette smoking is associated with an increased incidence of postoperative neuropathies, presumably because of the vasoconstrictive effects of nicotine.[12]
Brachial plexus injuries occur primarily in cardiothoracic procedures requiring median sternotomy. Shoulder braces used for head-down procedures have also been implicated as a cause of postoperative brachial plexus neuropathy, and this complication has also been described after some prone procedures.
Mechanisms for brachial plexus injury during median sternotomy include stretch or compression of the plexus during sternal separation, direct trauma from fractured 1st ribs, stretching related to internal mammary dissection, and trauma or hematoma related to internal jugular vein cannulation. Brachial plexus injuries constitute 20% of claims for nerve injury in the ASA Closed Claims Database (see Table 28-2 ).[1] A prospective study of patients undergoing open heart surgery found the incidence of these injuries to be as high as 4.9%.[7] Lower parts of the plexus are most often involved, producing painless motor deficits that usually resolve over 6 to 8 weeks.
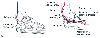 Figure 28-1
A, The retinaculum of
the cubital tunnel is depicted distinct from the aponeurosis of the flexor carpi
ulnaris (FCU) with which its distal margin blends. B,
The ulnar nerve and its primary blood supply in the proximal forearm, the posterior
ulnar recurrent artery, are superficial as they pass posteromedially to the tubercle
of the coronoid process. (Adapted from Warner MA: Perioperative neuropathies.
Mayo Clinic Proc 73:569, 571, 1998.)
Figure 28-1
A, The retinaculum of
the cubital tunnel is depicted distinct from the aponeurosis of the flexor carpi
ulnaris (FCU) with which its distal margin blends. B,
The ulnar nerve and its primary blood supply in the proximal forearm, the posterior
ulnar recurrent artery, are superficial as they pass posteromedially to the tubercle
of the coronoid process. (Adapted from Warner MA: Perioperative neuropathies.
Mayo Clinic Proc 73:569, 571, 1998.)
Shoulder braces placed close to the neck of patients undergoing noncardiothoracic procedures in a steep head-down (Trendelenburg) position may put pressure directly on the upper roots and trunks of the brachial plexus. Braces placed more laterally may put excessive traction on the brachial plexus by displacing the shoulder inferiorly. Coppieters and coworkers[9] studied the effect of the brachial plexus tension test (BPTT), a clinical equivalent of straight leg raising in the upper extremity, in asymptomatic male volunteers. Their results led them to strongly discourage the use of shoulder braces in positioning patients for surgery. Nonsliding mattresses were recommended when shoulder braces were thought absolutely required for a steep head-down tilt.
The prone position has also been implicated in brachial plexus injuries. Although theoretical advantages may support tucking the arms at the side in prone patients or turning the head to the side of an arm abducted up, no data support this idea. Brachial plexus injuries in prone patients are rare.
Ulnar neuropathy was formerly attributed to intraoperative compression or stretching because of the nerve's vulnerable position at the elbow where it passes around the medial epicondyle of the humerus and under the retinaculum of the cubital tunnel ( Fig. 28-1 ). The anatomic boundaries of the cubital tunnel are the floor (i.e., medial ligament of the elbow) and the roof (i.e., arcuate ligament, which extends from the medial epicondyle of the humerus to the medial aspect of the olecranon process of the ulna). The ulnar nerve then passes posteromedially to the tubercle of the coronoid process of the ulna; the arterial supply of the nerve in this area, the posterior recurrent ulnar artery, is very superficial. Anatomically, the tubercle of the coronoid process is 1.5 times larger in men and boys, who also are more likely to have a thicker cubital tunnel retinaculum. Women and girls have a much thicker layer of subcutaneous fat between the skin and the ulnar nerve at the cubital tunnel and the medial aspect of the coronoid process.[13]
An exhaustive retrospective study of the Mayo Clinic database for perioperative ulnar neuropathies occurring
Warner and coworkers[14] concluded that perioperative ulnar neuropathies might not be related to patient positioning for several reasons. Symptoms were not observed for more than 24 hours after the procedure in 57% of patients. Many of the neuropathies occurred in patients who did not receive general anesthesia. Bilateral ulnar neuropathies occurred in 9%. There were strong associations with several patient characteristics: male gender, very obese or very thin body habitus, and prolonged hospitalization (>14 days).
Two prospective studies further strengthened the suspicion that the injury associated with postoperative ulnar neuropathy does not usually occur intraoperatively. In the first, 1502 surgical patients were carefully assessed for neurologic signs and symptoms before and after surgery.[15] Warner and coworkers[15] found that seven patients (1 in 215) developed ulnar neuropathy perioperatively, but that none of the 7 was symptomatic within the first 2 postoperative days. The median onset of symptoms was the fourth postoperative day. Six of seven of the patients were men. In another prospective study of medical patients requiring no surgical procedures, 2 of 986 patients developed ulnar neuropathy.[16] It has become accepted that postoperative ulnar nerve palsy can occur without any apparent cause, even when positioning and padding of the patient's arms has been carefully done perioperatively. Postoperative ulnar nerve palsy is not always a preventable complication.[17]
The radial nerve may be injured where it wraps laterally around the middle of the humerus or laterally in its groove at the elbow. More muscular patients may be at higher risk because of limitations of extension of the elbow caused by hypertrophied biceps muscles and inflexible tendons. This may put the median or the anterior interosseous nerve on a greater stretch intraoperatively.[18] Transient median nerve carpal tunnel symptoms were identified in 27 of 1502 patients followed prospectively by Warner and coworkers[15] during the perioperative period. The anterior interosseous nerve contains no sensory fibers, but loss of it produces weakness of the thumb and index finger and a characteristic square pinch rather than an "O" on opposition of the thumb and index finger. Specific sensory and motor deficiencies caused by upper and lower extremity neuropathies are listed in Table 28-4 .
A large, retrospective study found motor neuropathies persisting more than 3 months in 55 patients among those undergoing 198,461 procedures in the lithotomy position, which is an incidence of 1 in 3608.[19] Forty-three of the 55 neuropathies involved the common peroneal nerve. Motor function of the affected nerve recovered in 43% of the patients within 1 year. Three risk factors were strongly associated with increased risk of developing a neuropathy in the lithotomy position: prolonged surgery (>3 hours), very thin body habitus, and recent cigarette smoking.
Warner and coworkers[20] also prospectively studied lower extremity neuropathies in 991 lithotomy patients. Fifteen patients (1.5%) developed neuropathies. The obturator, lateral femoral cutaneous, sciatic, and peroneal nerves were involved in five, four, three, and three patients, respectively. Fourteen of 15 patients presented with paresthesias; the symptoms resolved within 6 months in all but 1 patient. Prolonged positioning in the lithotomy position (>2 hours) and the extremes of body weight were found to contribute to an increased risk for lower extremity neuropathies after surgery in the lithotomy position in this prospective study.
Transient neurologic symptoms, also referred to as transient radicular irritation, can manifest as severe pain radiating down both legs after spinal anesthesia with lidocaine. The lithotomy position is associated with more frequent occurrences of this problem.[21]
The common peroneal nerve passes around the head of the fibula laterally, where it can be injured by compression by leg holders used to position patients in a lithotomy position ( Fig. 28-2 ). The sciatic nerve can be stretched by hyperflexion of the hip and extension of the knee in patients in a lithotomy position.
The femoral nerve can be injured by compression by abdominal wall retractors. The obturator nerve passes through the pelvis and is occasionally injured by surgical retractors placed deep below the pelvic brim. Excessive flexion can also injure the obturator nerve.
The lateral femoral cutaneous nerve is sensory only. Excessive flexion of the hip on the abdomen may occasionally cause compression leading to temporary neuropathies.
Warner has recommended early neurology consultation for patients with motor neuropathies; electromyographic (EMG) studies can be helpful to determine the location of the lesion and may provide information on the potential reversibility of any nerve deficit.[8] In contrast, sensory neuropathies often are transient, and initial reassurance might be all that is necessary.[8] [22] Since anesthesiologists often will be unable to provide long-term surveillance of patients who develop sensory deficits, it seems prudent to seek neurology consultation for sensory deficits lasting more than 5 days.
Eye complications are frustratingly common in patients who have undergone procedures in the prone position and
| Nerve | Motor Deficit | Sensory Deficit |
|---|---|---|
| Axillary | Loss of arm abduction by deltoid muscle | Deficit affects "arm-patch" area of the lateral shoulder. |
| Musculocutaneous | Loss of elbow flexion by biceps brachii (injury below the elbow yields sensory injury only) | Radial aspect of the volar forearm is affected. |
| Ulnar | A weak hand results from loss of function of many intrinsic hand muscles. Injuries at or above the elbow may produce weakness in the flexor digitorum profundus to ring and small fingers. Imbalance between interossei weakness (low ulnar nerve palsy) and digital flexor weakness (high ulnar nerve palsy) causes different degrees of ring and little finger "clawing." | The little finger and the ulnar (medial) half of the hand and ring finger are affected. The autonomous zone of sensory loss is the ulnar-palmar aspect of the little finger. |
| Radial | Loss of wrist and metaphalangeal joint extension results in wristdrop and fingerdrop. Lesions in the axilla also cause loss of elbow extension by the triceps brachii. | Radial aspect of the dorsal forearm is affected, continuing to the dorsum of the hand, thumb, and index and long fingers. The autonomous zone of sensory loss is in the region of the anatomic snuff box. |
| Median | Injury in the distal forearm (low median nerve palsy) results in impaired thumb opposition, abduction, and profound thenar atrophy. Lesions at or above the elbow (high median nerve palsy) may produce weakness in finger flexion and wrist flexion | Deficit affects the distal palm and palmar surfaces of the thumb and the index, long, and half of the ring fingers. The sole sensory innervation to the fingertips of the index and long fingers is affected. The autonomous zone is in the radial-palmar aspect of the index finger. |
| Anterior interosseous | Deficit causes loss of flexor pollicis longus and flexor digitorum profundus function of the index and possibly middle fingers. This results in an inability to form an "OK" sign. Loss of pronator quadratus function results in some weakness of pronation. | None |
| Femoral | Deficit causes loss of extension of the lower limb by the iliopsoas and quadriceps and gives rise to the purely sensory saphenous nerve (below). | Anterior surface of the thigh to the knee is affected. |
| Saphenous |
|
Medial surfaces of the knee, leg, and foot are affected. |
| Obturator | Deficit impairs adduction of the thigh. | Medial surface of the thigh is affected. |
| Sciatic | Deficit impairs hamstring function in knee flexion and all motor function below the knee. Incomplete lesions typically affect the peroneal division preferentially | Sensory components of the tibial and common peroneal nerves are affected. |
| Tibial | Deficit impairs plantar flexion of the foot by the gastrocnemius and soleus muscles and inversion by the posterior tibialis, and causes loss of toe flexion by flexor hallucis longus and flexor digitorum. | Sole of the heel and foot is affected. |
| Sural |
|
Lateral surface of foot is affected. |
| Common peroneal | Footdrop results from impaired ankle dorsiflexion. Deficit causes loss of eversion and toe extension. | Lateral surface of the leg and the dorsal surface of the foot are affected. |
| Lateral femoral cutaneous |
|
Upper anterolateral thigh is affected. |
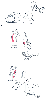 Figure 28-2
A, Lithotomy position,
showing leg placement. B and C,
Positioning is poor because the unpadded lithotomy leg holder can compress the common
peroneal nerve laterally or the saphenous nerve medially. (Adapted from
Martin JT: Lithotomy positions. Anesth Analg 1992;75:275 and from Smith BE: Obstetrics.
In Martin JT, Warner MA [eds]: Positioning in Anesthesia
and Surgery, 3rd ed. Philadelphia, WB Saunders, 1997, pp 47–70.)
Figure 28-2
A, Lithotomy position,
showing leg placement. B and C,
Positioning is poor because the unpadded lithotomy leg holder can compress the common
peroneal nerve laterally or the saphenous nerve medially. (Adapted from
Martin JT: Lithotomy positions. Anesth Analg 1992;75:275 and from Smith BE: Obstetrics.
In Martin JT, Warner MA [eds]: Positioning in Anesthesia
and Surgery, 3rd ed. Philadelphia, WB Saunders, 1997, pp 47–70.)
Corneal abrasions are most common, often involving the "down eye" in patients who were positioned prone with their heads turned to the side. One study put the prevalence at 0.17% for prone patients and found taping just after induction of anesthesia to be more effective than ophthalmic ointment used intraoperatively. [23] Although direct trauma can occur to the cornea, swelling of the dependent eye may be the mechanism causing the down eye to open slightly, allowing a slit-shaped area of cornea to dry. The patient with a corneal abrasion complains of eye pain and a foreign body sensation on awakening from anesthesia. Corneal abrasions usually heal quickly; the affected eye can be patched and antibiotic ointment applied to prevent bacterial infection that could lead to a corneal ulcer.
Pressure on the eyes should always be avoided. Small increases in pressure may occlude venous flow in the retina. If the pressure is slightly higher, arterial flow can be impaired and contribute to ischemia of the retina or optic nerve. Retinal artery occlusion usually manifests as a painless, monocular blindness with a pale edematous retina and a cherry-red spot at the fovea.
Ischemic optic neuropathy (ION) has been recognized as a serious complication of hemorrhage in cardiovascular, spinal, and other types of surgery, leading to blindness in one or both eyes postoperatively.[24] [25] The association between blood loss, hypotension, and blindness was mentioned by Hippocrates. ION is caused by infarction or ischemia of the anterior or posterior parts of the optic nerve. The blood supply to the posterior optic nerve is a watershed zone where delicate connections exist between the small arterial branches of the central retinal artery and outer pial vessels from branches of the ophthalmic artery.[24]
Many patients with ION do not have atherosclerosis and other cardiovascular risk factors; the complication has been reported in patients as young as 13 years. [25] In an extensive review in patients undergoing cardiopulmonary bypass, ION was diagnosed in 17 of 27,915 patients (0.06%).[26] Four cases occurred in 501,342 anesthetics (0.0008%) not involving cardiopulmonary bypass at the same institution.[27] None of these involved spinal surgery, but three cases of ION involving spinal surgery occurred at this institution in the first 4 years after the review.
The ASA Professional Liability Committee established a Registry for Postoperative Visual Loss (POVL Registry) in 1999 to collect data on this problem (www.ASAclosedclaims.org) (see Chapter 82 ). In the first 4 years, 79 cases were collected. In contrast to the literature cited earlier, 67% of the ASA POVL Registry cases involved prone spinal procedures, and 81% were caused by ION. The median age of the reported cases was 49; the age range was 19 to 73.
Other factors such as stretch and compression of the optic nerve due to orbital swelling in the prone patient may be involved in ION. Significant swelling often occurs in the orbits after prolonged procedures done in the
 Figure 28-3
Causes of perioperative eye complications are depicted.
Corneal abrasions are most common. Swelling or pressure can lead to retinal vein
or artery occlusion. Ischemic optic neuropathy is caused by infarction of the optic
nerve. Injuries to the optic chiasm can occur during pituitary surgery, and cortical
blindness can occur after some cardiac and neurosurgical procedures. (Adapted
from Williams EL, Hart WM, Tempelhoff R: Postoperative ischemic optic neuropathy.
Anesth Analg 80:1018–1029, 1995.)
Figure 28-3
Causes of perioperative eye complications are depicted.
Corneal abrasions are most common. Swelling or pressure can lead to retinal vein
or artery occlusion. Ischemic optic neuropathy is caused by infarction of the optic
nerve. Injuries to the optic chiasm can occur during pituitary surgery, and cortical
blindness can occur after some cardiac and neurosurgical procedures. (Adapted
from Williams EL, Hart WM, Tempelhoff R: Postoperative ischemic optic neuropathy.
Anesth Analg 80:1018–1029, 1995.)
Cortical blindness from hemorrhagic or embolic infarctions of the occipital lobe also occurs in the perioperative period, most commonly in patients after cardiac and neurosurgical procedures (see Fig. 28-3 ). Injuries or bleeding in the optic chiasm can lead to blindness after pituitary surgery.
|
|
|
|
|
|
|
|
|
|
|
|
|