|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Any of the following conditions may indicate the presence of ischemic heart disease: a history of viselike chest pain, with or without radiation to the inner aspect of the arm or neck; dyspnea on exertion, on exposure to cold, with defecation, or after eating (especially postmenopausal women who do not take estrogen); orthopnea; paroxysmal nocturnal dyspnea; nocturnal coughing; nocturia; previous or current peripheral or pulmonary edema; history of MI; family history of coronary artery disease; diagnosis of MI by ECG or elevated levels of enzymes; and cardiomegaly. Other patients who should be suspected of having ischemic heart disease include those who have diabetes, hypertension (especially if they are cigarette smokers or hyperlipemic),[31] [32] [333] left ventricular hypertrophy on ECG or echocardiogram,[77] [78] [334] [335] peripheral vascular disease (Lichtor JL and colleagues, personal communication),[335] [336] [337] [338] [339] [340] [341] [342] carotid bruits,[339] [340] [341] [342] [343] [344] [345] asymptomatic carotid artery occlusion,[339] [340] or unexplained tachycardia or fatigue.
The more difficult question to answer is how common ischemic heart disease is in asymptomatic patients or those with a normal ECG but predisposing conditions. The history appears to be the best indicator of coronary artery disease. Tomatis and coworkers[336] recorded coronary angiograms for "nearly all patients" who presented for aortoiliac reconstruction or resection of an abdominal aortic aneurysm. Of those with normal ECG results and histories not suggestive of coronary artery disease, 38% had stenosis of at least 50% in one or more coronary arteries, and 14% had stenosis of at least 75% in one or more coronary arteries. The percentages of patients with stenosis were the same for asymptomatic patients with abnormal ECG findings. However, a normal ECG result was not sensitive in ruling out significant stenosis when vascular disease was present: 44% of patients with normal ECGs and peripheral vascular disease had stenosis of at least 50% in one or more coronary arteries, and 30% had stenosis of at least 75%.
Hertzer and associates[337] [338] found angina and a history of myocardial disease to be reliable indicators of coronary artery disease. These investigators studied 1000 patients with peripheral vascular disease. Of the 500 patients with normal ECG results and no history of myocardial disease, 37% had narrowing of at least 70% in one or more coronary arteries. By contrast, of those suspected of having coronary artery disease because of history or ECG results (or both), 78% had narrowing of this same degree. In addition, patients who currently had angina had a 66% incidence of either severe correctable or severe inoperable coronary artery disease, whereas those who had peripheral vascular disease but no angina had an incidence of only 22.5%. For women, knowing the estrogen status appears to be crucial to interpreting other aspects of their cardiovascular history. [346] Thus, in addition to the symptoms just listed that are sought by routine questioning,[347] information about age, smoking, history of diabetes, cholesterol level, exercise tolerance, and estrogen status can all help predict the likelihood of coronary artery disease.
Are these data from 20 and 30 years ago still valid today? We believe so. Even though the average age of the typical surgical patient in the United States is now about 10 years higher than it was 25 years ago (Lichtor JL and colleagues, personal communication), the physiologic age (i.e., the RealAge) of the patients now being operated on is probably similar to that of patients 20 years ago.[26] [27] Therefore, although the age-adjusted mortality rates for coronary artery disease have decreased by approximately 45% and the age-adjusted mortality rate for all in-hospital surgical procedures has fallen by over 45%, aging of the surgical population has caused us to face the same risks that we faced 2 decades ago.
Although over 80% of all episodes of myocardial ischemia are "silent," [348] [349] patients with myocardial ischemia are not "silent." For example, even though the episodes of myocardial ischemia may have been silent, for all 165 patients studied by Rabkin and Horne[347] [350] who had ECG evidence of coronary artery disease, the patient's history gave an indication of the risk of coronary artery disease. This has also been the finding of our own studies[347] [351] and several others when physiologic age of 75 years or older has been added to the factors in a clinical history that would indicate myocardial ischemia. These indicators consist of the following: chest pain, typical or atypical; dyspnea on exertion or exposure to cold, with defecation, or after eating; nocturnal coughing; nocturia; previous or current peripheral or pulmonary edema; history of MI; a family history (especially sibling history) of coronary artery disease; cardiomegaly; abnormal ECG results; diabetes, hypertension; hyperlipemia; left ventricular hypertrophy; peripheral vascular disease; cigarette smoking; carotid artery bruits; peripheral vascular surgery; and unexplained tachycardia.
Several studies of asymptomatic carotid artery disease have shown very high perioperative mortality rates and life risk from associated ischemic heart disease.[339] [343] [344] [345] Barnes and Marszalek[340] found perioperative mortality rates of 18.2% and 15%, respectively, for patients with an asymptomatic carotid bruit or occlusive disease but a rate of only 2.1% for patients undergoing similar peripheral vascular procedures who did not have a carotid bruit.
|
|
Incidence of Stroke in Patients | |
|---|---|---|
| Study | With Bruits | Without Bruits |
| Ropper et al.[343] | 1/104 | 4/631 |
| Of those having vascular surgery | 1/37 | 4/130 |
| Barnes et al.[339] | 5/85 * | 0/364 |
| Perioperative deaths | 10.6% | 0.3% |
| Reed et al.[345] |
|
|
| CABG surgery; 54 strokes | 13/54 † | 4/54 † |
| CABG, coronary artery bypass graft. | ||
The important point to remember is that the history is the best indicator of coronary artery disease. In most series, the sensitivity and specificity of the history in indicating coronary artery disease range from 80% to 91% (Lichtor JL and colleagues, personal communication).[333] [335] [336] [337] [345] [350] [351] [353] [354] The history has higher sensitivity and specificity than most tests in indicating such disease (also see Chapter 25 for the definition of sensitivity and specificity). However, one test is gaining in popularity because it has been demonstrated to be easy to perform and to accurately predict perioperative morbidity.
Fowkes and colleagues[77] [355] [356] are testing a simple-to-measure index that would predict the degree of subclinical arterial aging that exists and the likelihood of subsequent cardiovascular events and death: the ratio of systolic BP in the posterior tibial artery of the ankle (as measured by Doppler) to systolic BP in the brachial artery (as measured by cuff). This index has predicted 8- and 10-year cardiovascular mortality in nonoperated patients: the incidence of cardiovascular death was 85% higher in those with an index of less than 0.7. The index is made even more powerful when combined with other historical factors. Patients who had an index of less than 0.9, were hypertensive, and currently smoked had a 43.8% risk of cardiovascular events in the next 5 years, as opposed to the 15.6% risk for those who had identical conditions but an index of 1.0 or higher.
Older's exercise index ("aerobic threshold") seems to have the same goal[357] [358] —to predict the degree of sub-clinical arterial aging. The RealAge system attempts to do the same thing with its measurements but uses a much broader range of modifiable factors.[26] [27]
However, the key payoff would be the demonstration that using such tests would motivate patients to modify health-related habits, which in turn would improve their index values. This result would then have to be correlated with beneficial effects on perioperative cardiovascular events. (Examples of changes in health-related habits include food choices, smoking and smoke inhalation, increased physical activity, consumption of antioxidants, use of aspirin, use of β-blocking drugs, use of statins, weight reduction, BP control, anti-inflammatory treatments, and flossing of teeth.) Although an increasing body of data[26] [27] [342] [359] [360] seem to predict that such changes would occur, they have not yet been confirmed. Such a study would be incredibly difficult to execute successfully, but would any study be more worthwhile?
The presence of coronary artery disease, its severity, the time of the most recent myocardial tissue death, the arteries affected, ventricular function and reserve, and the complications and treatment of the disease are important information to the anesthesiologist. These variables influence the manner in which anesthesia is given and, in fact, may determine whether anesthesia and surgery should be postponed.
Numerous epidemiologic studies[189] [322] [323] [361] [362] [363] [364] [365] [366] [367] [368] [369] [370] ( Table 27-24 ) (but none in the last 9 years) have shown that if a previous MI and subsequent surgery are separated by less than 6 months, the perioperative reinfarction rate is 5% to 86% (1.5 to 10 times higher than the value when previous MI and subsequent surgery are separated by more than 6 months), and the mortality rate is 23% to 86%. After 6 months, the perioperative reinfarction rate seems to stabilize at 2% to 6%. An investigation by Schoeppel and colleagues[368] in which a small number of patients were studied produced a 0% mortality rate in the first year after infarction but a perioperative reinfarction rate of 16.7% and a mortality rate of 67% in patients experiencing perioperative reinfarction. Because the incidence and timing of perioperative reinfarction in the study by Schoeppel and coworkers differ so much from those variables in the other studies listed in Table 27-24 , we have placed little emphasis on that study. However, for all 12 of the studies listed in Table 27-24 , the overall reinfarction rates are similar.[189] [315] [316] [317] [320] [321] [322] [323] [351] [353] [354] [361] [362] [363] [364] [365] [366] [367] [368] [369] [370] [371] These data do not include patients who underwent immediate
| Time from MI Operation (mo) | Arkins et al[361] 1963: Mort | Topkins and Artusio[362] 1959–1963 | Fraser et al[363] 1960–1964 Mort | Tarhan et al[364] 1967–1970 | Sapala et al[365] 1970–1974 | Steen et al[366] 1970 | von Knorring[370] 1981 | Goldman et al[367] 1975–1976 | Eerola et al[327] 1970–1974 | Schoeppel et al[368] 1980 | Rao et al[190] 1973–1976 | Rao et al[190] 1976–1982 | Shah et al[371] 1983–1986 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reinf | Mort | Reinf | Mort | Reinf | Mort | Reinf | Mort | Reinf | Mort | Reinf | Mort | Reinf | Mort | Reinf | Mort | Reinf | Mort | Reinf | Mort | Reinf | Mort | |||
| 0–3 | 40% |
|
* | 38% | 37% | * | 86% | 86% | 27% |
|
|
* | 4.5% | 23% | 8% | 8% | 0% | 0% | 30% | * | 5.8% | * | 4.3% |
|
|
|
(11/27) | 54.5% |
|
(19/38) | (3/8) |
|
(6/7) | (6/7) | (2/18) |
|
25% |
|
(1/22) | (5/22) | (1/12) | (1/12) | (0/1) | (0/1) | (4/11) |
|
(3/52) |
|
(1/23) |
|
| 4–6 | * | (12/22) | * | * | 16% | * |
|
|
11% |
|
(4/16) |
|
|
5.9% | 5.9% | 0% | 0% | 0% | 26% | * | 2.3% | * | 0% |
|
|
|
|
|
|
|
(3/19) |
|
|
|
(2/18) |
|
|
|
|
(1/12) | (1/17) | (0/1) | (0/8) | (0/8) | (8/3) |
|
(2/36) |
|
(0/18) |
|
| 7–12 | * | 25.0% | * | * | 5% | * |
|
|
|
|
18% | * |
|
|
|
|
|
|
5% | * | 1.0% | * |
|
|
|
|
|
(9/36) |
|
|
(2/42) |
|
|
|
|
|
(2/11) |
|
0% |
|
|
|
|
|
(6/127) |
|
(1/104) |
|
|
|
| 13–18 | * |
|
* | * | 4% | * |
|
|
|
|
|
|
(0/13) | 8% |
|
|
|
|
5% |
|
1.6% | * |
|
|
|
|
|
22.4% |
|
|
(1/27) |
|
|
|
|
|
|
|
|
(1/13) |
|
|
0% | 0% | (6/114) |
|
(4/258) |
|
|
|
| 19–24 | * | (11/49) | * | * | 4% | * | 5.7% | 1.9% |
|
|
|
|
|
|
|
|
(0/10) | (0/10) |
|
|
|
|
5.7% |
|
|
|
|
|
|
|
(1/21) |
|
(9/159) | (3/159) | 5.4% |
|
11% |
|
|
|
4.9% | 12% |
|
|
|
|
|
|
(10/74) |
|
| 25–36 | * | 5.9% | * | * |
|
* |
|
|
(30/544) |
|
(10/89) | * | 3.3% | 3.3% | (4/82) | (1/82) |
|
|
|
* |
|
* |
|
|
|
|
|
(3/51) |
|
|
5% |
|
|
|
|
|
|
|
(2/66) | (2/66) |
|
|
|
|
5% |
|
1.7% |
|
|
|
| >36 | * | 1.0% | * | * | (11/232) |
|
|
|
|
|
|
|
|
|
|
|
0% | 0% | (4/81) |
|
(4/235) |
|
|
|
|
|
|
(5/493) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(0/26) | (0/26) |
|
|
|
|
|
|
| Unknown | * | 42.8% | * | * | 5.6% | * |
|
|
|
|
22% | * |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(3/7) |
|
|
(7/73) |
|
|
|
|
|
(9/41) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total patients with MI | * | 6.5% | 4.7% | * | 6.6% | 3% | 9% | 5.4% | 6.1% | 4.2% | 15.9% | 4% |
|
8.9% |
|
|
5.7% | 3.8% | 7.7% | 4.1% | 1.9% | 0.7% | 4.7% | 1.1% |
|
|
|
(43/658) | (31/658) |
|
(28/422) | (15/422) | (15/166) | (9/166) | (36/587) | (25/587) | (25/157) | (7/157) |
|
(9/109) |
|
|
(3/53) | (2/53) | (28/364) | (15/364) | (14/733) | (5/733) | (13/275) | (3/275) |
| MI, myocardial infarction: Mort, mortality: Reinf, reinfarction. | ||||||||||||||||||||||||
The study conducted by Rao and associates[189] deserves special comment because it proposes to show a vast decrease in the perioperative reinfarction rate with the use of modern monitoring techniques. Rates of perioperative reinfarction and mortality were compared in two groups of patients who had previous MIs and were operated on at two different time periods: 364 patients between 1973 and 1976 and 733 patients between 1976 and 1982. Rao and coworkers attribute the reduction in the overall perioperative reinfarction rate (from 7.7% to 1.9%) and in each time period (i.e., from 36% to 5.8% when surgery occurs within 3 months of a previous MI) to invasive monitoring and rapid treatment of cardiovascular variables when values deviated from normal. These two practices apply to both the intraoperative period and the first 72 postoperative hours. (The 72-hour period may be critical because virtually all 12 studies and others showed that reinfarction was most likely to occur 24 to 96 hours after surgery[189] [322] [323] [361] [362] [363] [364] [365] [366] [367] [368] [369] [370] [371] [372] [373] [374] [375] [376] [377] [378] [ Table 27-25 ].) Most of the reduction in the perioperative reinfarction rate applied to patients older than 65 years. (MI makes the physiologic age or risk of death equivalent to that of someone 3 to 17 years older, depending on the location of the infarction and the decrease in left ventricular ejection fraction.[26] [27] )
An editorial evaluating the study by Rao and colleagues stated that the use of historical controls may have biased the conclusions. Moreover, a different patient mix, improved skills of the surgeon and anesthetist, and other unidentified or unmeasured changes over time may have contributed to the reduction in the reinfarction rate.
Despite the evidence of an increased risk for surgery in patients with a recent MI, Rivers and associates demonstrated relatively low cardiac morbidity (20%) in a cohort of 30 patients requiring urgent or emergency vascular procedures in the first 6 weeks after an MI.[377] Four post-operative deaths (three cardiac related) and two nonfatal reinfarctions occurred. Cardiac complications did not correlate with age, interval from MI to surgery within the initial 6 weeks, type of anesthesia, or complexity of the operation.
Normalization of hemodynamics by pulmonary artery catheter monitoring and 3 days in an ICU also appears to reduce perioperative morbidity in vascular surgery patients, a group at high risk for postoperative cardiac morbidity. In a randomized controlled trial, Berlauk and coworkers[378] examined that intervention scheme, which was similar to the one investigated by Rao and associates, [189] for patients undergoing vein graft bypass for limb salvage from 1986 to 1990. Forty-five patients were randomly assigned to group 1 (i.e., pulmonary artery catheterization performed ≥12 hours before surgery, followed by treatment for hemodynamic optimization). The 44 other patients were randomly assigned to either group 2 (23 patients; i.e., pulmonary artery catheterization within 3 hours of surgery, followed by a shorter treatment for
Berlauk and colleagues reported that patients undergoing less
intensive treatment (group 3) were significantly more likely to have intraoperative
complications (tachycardia, hypotension, arrhythmia) than were patients in group
1. The overall incidence of postoperative complications (renal failure, CHF, MI,
graft thrombosis, death) was 18% for group 1, 13% for group 2, and 43% for group
3. Half the complications in group 3 were attributable to early graft thrombosis
and accounted for most of the difference in complication rates. The authors conjectured
that the increased incidence of thrombosis was due to
|
|
|
|
Postoperative | ||||
|---|---|---|---|---|---|---|---|
| Investigators | Year Published | Examined Prospectively | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 |
| Plumlee and Boettner[372] | 1972 | No | 11/24 | 3/24 | 1/24 | 2/24 |
|
| Tarban et al[364] | 1972 | No |
|
14/71 | 8/71 | 22/71 | 13/71 |
| Rao et al[190] | 1983 | No |
|
8/28 | 7/28 | 10/28 | 3/28 |
|
|
|
No |
|
3/14 | 4/14 | 7/14 |
|
| Becker and Underwood[373] | 1987 | ? | 11/28 | 6/28 | 9/28 | 0/28 | 1/28 |
| Total (4 studies) |
|
|
22 | 34 | 29 | 41 | 17 |
This study had other deficiencies that preclude definitive advice on ways of reducing morbidity in patients with
Treadmill exercise testing, bicycle ergometry, dipyridamolethallium
imaging, dobutamine stress echocardiography,
| Test | Studies (N) | RR (95% CI) | Cost (U.S. $) |
|---|---|---|---|
| DTS | 11 | 6.3 (3.0–13.1) | 1200–1400 |
| RNV | 5 | 3.8 (1.5–9.4) | 500–600 |
| AECG | 6 | 3.7 (2.0–7.1) | 300–550 |
| DSE | 3 | 19.0 (5.9–60.8) | 1000–1200 |
| AECG, ambulatory electrocardiography; CI, confidence interval; DSE, dobutamine stress echocardiography; DTS, dipyridamole-thallium scintigraphy; RNV, estimation of ejection fraction by radionuclide ventriculography; RR, relative risk. | |||
ECG criteria for myocardial ischemia during or after exercise consist of at least 1 mm of J-point depression with downsloping or horizontal ST segments; slowly upsloping ST-segment depression, defined as 2 mm of ST depression measured 80 milliseconds from the J point; and ST-segment elevation[345] ( Fig. 27-11 ). Other responses to treadmill testing that are predictive of severe multivessel or left main stem coronary artery disease include ST-segment depression exceeding 2.5 mm, serious ventricular arrhythmias at low heart rates or early (first 3 minutes) onset of ischemic ST-segment depression, or prolonged duration of the ischemic ST-segment depression in the post-test recovery period (>8 minutes).[353]
Non-ECG responses to treadmill testing that predict severe coronary artery disease include low achieved heart rates (≤120 beats/min), systolic hypotension (decrease of >10 mm Hg) in the absence of hypovolemia or antihypertensive medications, rise in diastolic BP to higher than 110 mm Hg, and an inability to exercise beyond 3 minutes. The treadmill test responses predictive of severe multivessel or left main stem coronary artery disease (or both) are listed in Table 27-27 . Clearly, however, the response to the test must be interpreted in light of the patient's history and with the knowledge that it is more predictive for men than women[353] ( Table 27-28 ).
 Figure 27-10
Relative risks and confidence intervals resulting from
the use of four preoperative tests to predict adverse outcomes after vascular surgery,
as reported by various studies. Names at the left refer to the first author of studies
reviewed by Mantha and colleagues.[387]
Broken
vertical lines represent combined relative risks, as assessed by each
test. AECG, ambulatory electrocardiography; DSE, dobutamine stress echocardiography;
DTS, dipyridamole-thallium scintigraphy; RNV, estimate of ejection fraction by radionuclide
ventriculography. (Adapted from Mantha S, Roizen MF, Barnard J, et al:
Relative effectiveness of preoperative noninvasive cardiac evaluation tests on predicting
adverse cardiac outcomes following vascular surgery: A meta-analysis. Anesth Analg
79:422, 1994.)
Figure 27-10
Relative risks and confidence intervals resulting from
the use of four preoperative tests to predict adverse outcomes after vascular surgery,
as reported by various studies. Names at the left refer to the first author of studies
reviewed by Mantha and colleagues.[387]
Broken
vertical lines represent combined relative risks, as assessed by each
test. AECG, ambulatory electrocardiography; DSE, dobutamine stress echocardiography;
DTS, dipyridamole-thallium scintigraphy; RNV, estimate of ejection fraction by radionuclide
ventriculography. (Adapted from Mantha S, Roizen MF, Barnard J, et al:
Relative effectiveness of preoperative noninvasive cardiac evaluation tests on predicting
adverse cardiac outcomes following vascular surgery: A meta-analysis. Anesth Analg
79:422, 1994.)
 Figure 27-11
Electrocardiographic (ECG) criteria for myocardial ischemia
consist of 1 mm or more of J-point depression with downsloping or horizontal ST segments;
slowly upsloping ST-segment depression, defined as 2 mm of ST depression measured
80 milliseconds from the J point; and ST-segment elevation. Whereas ST-segment depression
indicates nontransmural ischemia, ST-segment elevation often connotes more severe
degrees of ischemia reflecting transmural injury. The structure of the ST-segment
slope is predictive of the severity of coronary disease shown angiographically, with
downsloping ST depression indicating severe two- and three-vessel coronary artery
disease more often than either horizontal or slowly upsloping ST depression does
and ST-segment elevation indicating high-grade, usually proximal arterial obstruction
in patients without previous myocardial infarction. (Reproduced with permission
from Goldschlager N: Use of the treadmill test in the diagnosis of coronary artery
disease in patients with chest pain. Ann Intern Med 97:383, 1982.)
Figure 27-11
Electrocardiographic (ECG) criteria for myocardial ischemia
consist of 1 mm or more of J-point depression with downsloping or horizontal ST segments;
slowly upsloping ST-segment depression, defined as 2 mm of ST depression measured
80 milliseconds from the J point; and ST-segment elevation. Whereas ST-segment depression
indicates nontransmural ischemia, ST-segment elevation often connotes more severe
degrees of ischemia reflecting transmural injury. The structure of the ST-segment
slope is predictive of the severity of coronary disease shown angiographically, with
downsloping ST depression indicating severe two- and three-vessel coronary artery
disease more often than either horizontal or slowly upsloping ST depression does
and ST-segment elevation indicating high-grade, usually proximal arterial obstruction
in patients without previous myocardial infarction. (Reproduced with permission
from Goldschlager N: Use of the treadmill test in the diagnosis of coronary artery
disease in patients with chest pain. Ann Intern Med 97:383, 1982.)
| Electrocardiographic responses |
| ST-segment response |
| Downsloping |
| Elevation |
| ST-segment depression exceeding 2.5 mm |
| Serious ventricular arrhythmias occurring at low heart rates (120–130 beats/min) |
| Early onset (first 3 min) of ischemic ST-segment depression of elevation |
| Prolonged duration in the post-test recovery period (≥8 min) of ischemic ST-segment depression |
| Nonelectrocardiographic criteria |
| Low achieved heart rate (≤120 beats/min) |
| Hypotension * (≥10-mm Hg fall in systolic blood pressure) |
| Rise in diastolic blood pressure (≥110–120 mm Hg) |
| Low achieved rate-pressure product (≤15,000) |
| Inability to exercise beyond 3 min |
| Modified from Goldschlager N: Use of the treadmill test in the diagnosis of coronary artery disease in patients with chest pain. Ann Intern Med 97:383, 1982. |
An inability to increase the heart rate (cardiac risk index [CRI]) above 90 beats/min after supine bicycle exercise for 2 minutes at 50 rpm has been shown to predict perioperative MI with 80% sensitivity. This test is claimed by its investigators to have better sensitivity (80%) with a small sacrifice in specificity (53%) than the Goldman CRI (sensitivity, 60%; specificity, 64%). This same group recommended this test for geriatric patients at low risk (according to the Goldman CRI) to identify the "falsenegatives" of the Goldman CRI or for geriatric patients at risk for perioperative MI. Limitations of this test, however, are recognized in patients with impaired joint mobility, dementia, muscle weakness, claudication, or exertional angina.[376] Ejection fractions greater than 50% (and normal left ventricular size on plain chest radiographs) predict good perioperative cardiac function and survival.[390] [391]
| Clinical Characteristics | Prevalence of Coronary Artery Disease (%) | Predictive Accuracy of a Positive Test (%) | Predictive Accuracy of a Negative Test (%) |
|---|---|---|---|
| Asymptomatic | 5 | 10–20 | 95–100 |
| Noncardiac chest pain | 10–25 | 45–50 | 80–85 |
| Cardiac chest pain |
|
|
|
| Atypical, or probable, angina | 50–70 | 80–85 | 65–70 |
| Typical, or definite, angina | 85–95 | 95–100 | 50–55 |
| Modified from Goldschlager N: Use of the treadmill test in the diagnosis of coronary artery disease in patients with chest pain. Ann Intern Med 97:383, 1982. | |||
For dipyridamole-thallium scanning, patients received dipyridamole (0.56 mg/kg) intravenously over a 4-minute period while their heart rate, BP, and ECG were monitored. After an additional 2 minutes, when the effect of dipyridamole was considered maximal, 2 mCi of thallium 201 was administered intravenously. Five minutes after the administration of thallium, initial images were taken for 8 consecutive minutes. Delayed images were obtained 3 hours later. Anterior, 45-degree, and 70-degree left anterior oblique projections were taken each time. Dipyridamole causes vasodilation and an increase in coronary blood flow. Because stenotic vessels cannot dilate normally, areas of myocardium supplied by them will take up less thallium during scanning than will areas supplied by normal vessels and will show up as filling defects on immediate images. On late images, after the vasodilation has resolved, thallium is redistributed to these previously underperfused areas.
Dipyridamole infusion resulted in an increase in heart rate of 17 beats/min, a decrease in systolic BP of 17 mm Hg, and chest pain in 30% of patients, which was reversible with 125 mg of aminophylline intravenously. ECGs were examined for ischemic changes, defined as 1-mm or more horizontal or downsloping ST-segment depression. Myocardial regions on initial and delayed images were graded as being normal, as showing fixed deficits (without redistribution), or as showing one or more segments with thallium redistribution (with or without evidence of fixed deficits). Initial studies[383] [384] [392] (reviewed by Mantha and colleagues[387] ) have shown this test to be valuable in further stratifying high risk, as assessed by five clinical factors (CHF, diabetes mellitus, history of ventricular ectopy, angina, and history of MI). Of patients with thallium redistribution, 45% had a perioperative MI, whereas 7% of patients without redistribution had a perioperative MI (P = .001). Dipyridamole-thallium scanning has been suggested to be valuable in stratifying patients with intermediate risk, as assessed by five clinical factors (Q waves on resting ECG, history of ventricular ectopy, diabetes, age >70 years, and angina) that were identified by logistic regression on several clinical findings and Goldman CRIs and Dripp classes. Fletcher and colleagues[392] found the test superior to clinical assessment in identifying high-risk patients for further angiographic study, prophylactic CABG surgery, or intensive intraoperative and
Twenty-four- to 48-hour Holter monitoring of intermediate-risk patients has been shown to be effective in detecting asymptomatic ischemia and has an excellent negative predictive value and a fair positive predictive value for postoperative cardiac complications (see Fig. 27-10 and Table 27-26 ). Preoperative ischemia increases the risk for intraoperative ischemia threefold to fourfold. Raby and colleagues[385] studied 176 vascular surgery patients who were asymptomatic for ischemic cardiac disease and found preoperative ischemia on Holter monitoring to be an independent factor with high correlation for significant postoperative cardiac complications (e.g., infarction, unstable angina, and ischemic pulmonary edema). In this series, 24- to 48-hour Holter monitoring detected two of three patients with positive stress test results and four patients with postoperative cardiac complications who did not have a history suggestive of ischemic heart disease. With an overall sensitivity of 92%, specificity of 88%, positive predictive value of 38%, and negative predictive value of 99%, Holter monitoring may be the test of choice for evaluating intermediate-risk patients who have no suggestive history or for patients who are unable to undergo an exercise stress test. Importantly, other investigators have demonstrated the value of silent ambulatory ECG monitoring, although the negative predictive values have not been as high as originally reported. Fleisher and colleagues[7] demonstrated a similar predictive value of dipyridamole-thallium imaging and ambulatory ECG monitoring; however, the quantity of silent ischemia could not be used to identify patients at greatest risk who might benefit from further testing and coronary revascularization. Limitations of this method are seen in patients with left bundle branch block, left ventricular hypertrophy, strain, and ST-T changes with digoxin because of the difficulties in analyzing ST-segment changes.
Studies of visual interpretation of the coronary angiogram suggest that the physiologic effects of most coronary artery obstruction cannot be determined accurately by conventional angiographic approaches.
Dobutamine stress echocardiography has also been tested for its ability to predict adverse cardiac outcome in patients undergoing vascular surgery. [387] [394] [395] [396] [397] For this test, an abnormal result or a positive result consists of new regional wall motion abnormalities or worsening of existing regional wall motion abnormalities on echocardiography during infusion of dobutamine. A negative result consists of the absence of any change on echocardiography during dobutamine infusion. This test has high predictive value for adverse cardiac events (although the findings of Mantha and coworkers[387] are limited to only three studies of dobutamine stress echocardiography for this purpose). The relative risk (95% confidence interval [CI]) of an adverse outcome predicted by the test was 19 (5.9 to 60.8) (see Fig. 27-10 and Table 27-26 ), which suggests that this test is very "effective." It is interesting to note that unlike the other studies, the study of Mantha and colleagues[387] found that the predictive value increased over the time period that the test was available. Boersma and associates[397B] assessed the value of dobutamine stress echocardiography with respect to the extent of wall motion abnormalities and the ability of preoperative β-blocker treatment to attenuate risk in patients undergoing major aortic surgery. They assigned 1 point for each of the following characteristics: age older than 70 years, current angina, MI, CHF, previous cerebrovascular disease, diabetes mellitus, and renal failure. As the total number of clinical risk factors increases, perioperative cardiac event rates also increase.
Studies have concentrated on the identification and stratification of risk in the intermediate-risk group of patients who would benefit most from a preoperative cardiac workup and risk assessment. Goldman and colleagues[367] prospectively studied 1001 patients older than 40 years undergoing a broad variety of emergency and elective surgery. Multiple regression analysis of the data showed that nine factors independently correlated with the development of life-threatening or fatal cardiac complications: (1) the presence of an S3 gallop, jugular vein distention, or CHF; (2) a history of MI in the preceding 6 months; (3) a cardiac rhythm other than sinus; (4) the occurrence of more than five PVCs per minute; (5) performance of intraperitoneal, intrathoracic, or aortic surgery; (6) patient age older than 70 years; (7) significant valvular aortic stenosis; (8) emergency surgery; and (9) poor general medical condition. On the basis of this study, the investigators developed a method of computing a CRI ( Table 27-29 ). This study suggests that the history and physical examination account for 29 of 53 points in assessing the cardiac risk of the general surgical population and that the initial history and physical examination permit selection of intermediate-risk patients for further evaluation. Eagle and colleagues[383] studied 200 vascular surgery patients, a population with a high incidence of cardiovascular disease, and by logistic regression, found the following to be independent risk factors: Q waves on resting ECG, history of ventricular ectopy, diabetes, age older than 70 years, angina, and CHF.
One of the most recent clinical risk indices was developed by
investigators at the Brigham and Women's Hospital, who studied 4315 patients aged
50 years who were undergoing elective major noncardiac procedures in a tertiary care
teaching hospital.[400]
Six independent predictors
of complications were identified and included in a revised CRI: high-risk type of
surgery, history of ischemic heart disease, history of CHF, history of cerebrovascular
disease, preoperative treatment with insulin,
| Criteria | Points |
|---|---|
| 1. History |
|
| a. Age >70 yr | 5 |
| b. MI in previous 6 mo | 10 |
| 2. Physical examination |
|
| a. S3 gallop or JVD | 11 |
| b. Important VAS | 3 |
| 3. Electrocardiogram |
|
| a. Rhythm other than sinus of PACs on last preoperative ECG | 7 |
| b. >5 PVCs/min documented at any time before surgery | 7 |
| 4. General status |
|
| PO2 <60 or PCO2 >50 mm Hg, K <3.0 or HCO3 <20 mEq/L, BUN>50 or Cr>3.0 mg/dL, abnormal AST, signs of chronic liver disease, or patient bedridden from noncardiac causes |
|
| 5. Surgery |
|
| a. Intraperitoneal, intrathoracic, or aortic surgery | 3 |
| b. Emergency surgery | 4 |
| Total possible points | 53 |
| AST, asparate transaminase; BUN, blood urea nitrogen; Cr, creatinine; ECG, electrocardiogram; HCO3 , bicarbonate; JVD, jugular vein distention; K, potassium; MI, myocardial infarction; PACs, premature atrial contractions; PCO2 , partial pressure of carbon dioxide; PO2 , partial pressure oxygen; PVCs, premature ventricular contractions; VAS, valvular aortic stenosis. | |
| Modified from Goldman L, Caldera DL, Nussbaum SR, et al: Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 297:845, 1977. Risk calculations from Goldman et al.,[367] Detsky et al.,[375] and Jeffrey et al.[398] | |
| Factors That Make a Difference in Outcome |
| High-risk surgery |
| History of ischemic heart disease |
| History of congestive heart failure |
| History of cerebrovascular disease |
| Preoperative treatment with insulin |
| Preoperative serum creatinine level greater than 2.0 mg/dL |
| Rates of major cardiac complications if you had zero, one, two, or three of these factors were 0.4% to 0.5%, 0.9% to 1.3%, 4% to 7%, and 9% to 11%, respectively, after elective major noncardiac surgery. See the text for details. |
| From Lee TH, Marcantonio ER, Mangione CM, et al: Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 100:1043–1049, 1999. |
The Cleveland Clinic group has established a clinical severity scoring system to characterize risk before coronary artery bypass surgery[401] ( Table 27-31 and Fig. 27-12 ). This system predicts cardiovascular morbidity and gives stratification points for the risk of an adverse cardiovascular outcome in patients with preoperative renal insufficiency, diabetes, age, anemia, COPD, mitral valve insufficiency, aortic valve stenosis, cerebrovascular disease, and surgery performed on an emergency basis—many factors found by Goldman and colleagues to affect outcome adversely. Therefore, diseases that impinge on the cardiovascular system, such as renal insufficiency and diabetes, have important effects in predicting cardiovascular morbidity. What is the role of preoperative or preprocedure treatment of these conditions?
The American College of Cardiology/American Heart Association Task Force on Perioperative Cardiovascular Evaluation for Noncardiac Surgery used data such as those just presented, combined with expert opinion, to propose a series of algorithms for preoperative evaluation of such patients ( Table 27-32 , Table 27-33 , and Table 27-34 and Fig. 27-13A and Fig. 27-13B ). [10] They did this because of the importance of a surgical event to a patient's long-term prognosis and because surgery may present the first opportunity for long-term evaluation, risk management, and motivation of the patient. First, the clinician must evaluate the urgency of the surgery and the appropriateness of a formal preoperative assessment. Next, it should be determined whether the patient has undergone a previous coronary revascularization procedure or coronary evaluation. Patients with unstable coronary syndromes should be identified and appropriate treatment instituted. Finally, the decision to undergo further testing depends on the interaction of clinical risk factors, surgery-specific risk, and functional capacity. High clinical risk markers include acute coronary syndromes and severe valvular disease. Intermediate predictors of increased risk are factors that have been associated with higher
| Preoperative Factors | Score | Morbidity Odds Ratio |
|---|---|---|
| Surgery performed on an emergency basis | 6 | 9.0 |
| Serum creatinine levels |
|
|
| 141–167 µmol/L (1.6–1.8 mg/dL) | 1 |
|
| ≥168 µmol/L (≥1.9 mg/dL) | 4 | 2.8 |
| Severe left ventricular dysfunction | 3 | 2.1 |
| Reoperation | 3 | 2.5 |
| Operative mitral valve insufficiency | 3 | 2.6 |
| Age |
|
|
| 65–74 yr | 1 | 1.5 |
| ≥75 yr | 2 | 1.9 |
| Previous vascular surgery | 2 | 1.4 |
| Chronic obstructive pulmonary disease | 2 | 1.5 |
| Anemia (hematocrit ≤34%) | 2 | 1.6 |
| Operative aortic valve stenosis | 1 | 1.5 |
| Weight ≤65 kg | 1 | 1.5 |
| Diabetes, taking oral or insulin therapy | 1 | 1.5 |
| Cerebrovascular disease | 1 | 1.4 |
| Modified from Higgins TL, Estafanous FG, Loop FD, et al: Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. JAMA 267:2344, 1992. | ||
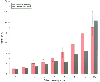 Figure 27-12
The observed morbidity after coronary artery bypass surgery
versus the morbidity predicted by a clinical severity scoring system that evaluated
various pertinent preoperative conditions. Morbidity was lower than the predicted
99.4% confidence interval at scores 2, 5, 6, and 7 to 9. The model may not be validated
prospectively because of changes in clinical management that could result in complications.
The vertical lines at top of the bars
indicate confidence intervals. (Redrawn from Higgins TL, Estafanous FG,
Loop FD, et al: Stratification of morbidity and mortality outcome by preoperative
risk factors in coronary artery bypass patients. A clinical severity score. JAMA
267:2344, 1992.)
Figure 27-12
The observed morbidity after coronary artery bypass surgery
versus the morbidity predicted by a clinical severity scoring system that evaluated
various pertinent preoperative conditions. Morbidity was lower than the predicted
99.4% confidence interval at scores 2, 5, 6, and 7 to 9. The model may not be validated
prospectively because of changes in clinical management that could result in complications.
The vertical lines at top of the bars
indicate confidence intervals. (Redrawn from Higgins TL, Estafanous FG,
Loop FD, et al: Stratification of morbidity and mortality outcome by preoperative
risk factors in coronary artery bypass patients. A clinical severity score. JAMA
267:2344, 1992.)
| Degree | Clinical Predictors |
|---|---|
| Major | Unstable coronary syndromes |
|
|
Recent myocardial infarction * with evidence of ischemic risk, as determined by clinical symptoms or noninvasive study |
|
|
Unstable or severe † angina (Canadian class III or IV) ‡ |
|
|
Decompensated congestive heart failure |
|
|
Significant arrhythmias |
|
|
High-grade atrioventricular block |
|
|
Symptomatic ventricular arrhythmias in the presence of underlying heart disease |
|
|
Supraventricular arrhythmias with uncontrolled ventricular rate |
|
|
Severe valvular disease |
| Intermediate | Mild angina pectoris (Canadian class I or II) |
|
|
Previous myocardial infarction, as determined by history or pathologic Q waves |
|
|
Compensated or previous congestive heart failure |
|
|
Diabetes mellitus |
| Minor | Advanced age |
|
|
Abnormal electrocardiogram (left ventricular hypertrophy, left bundle branch block, ST-T abnormalities) |
|
|
Rhythm other than sinus (e.g., atrial fibrillation) |
|
|
Low functional capacity (e.g., inability to climb one flight of stairs with a bag of groceries) |
|
|
History of stroke |
|
|
Uncontrolled systemic hypertension |
| From Eagle KA, Berger PB, et al: ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542–553, 2002, with permission. | |
| Metabolic Equivalents (METs) | Activity |
|---|---|
| 1 MET | Can you take care of yourself? |
|
|
Can you eat, dress, and use the toilet? |
|
|
Can you walk indoors around the house? |
|
|
Can you walk a block or two on level ground at 2 to 3 mph (3.2 to 4.8 km/hr)? |
| 4 METs | Can you climb a flight of stairs or walk up a hill? |
|
|
Can you walk on level ground at 4 mph (6.4 km/hr)? |
|
|
Can you run a short distance? |
|
|
Can you do heavy work around the house such as scrubbing floors or moving heavy furniture? |
|
|
Can you participate in moderate recreational activities such as golfing, bowling, dancing, doubles tennis, or throwing a baseball or football? |
| 10 METs | Can you participate in strenuous sports such as swimming, singles tennis, football, basketball, or skiing? |
| From Eagle KA, Berger PB, et al: ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542–553, 2002, with permission. | |
| Cardiac Risk * | Noncardiac Surgical Procedure |
|---|---|
| High reported cardiac risk (often >5%) | Emergency major operations, particularly in the elderly |
|
|
Aortic and other major vascular procedures |
|
|
Peripheral vascular procedures |
|
|
Anticipated prolonged surgical procedures associated with large fluid shifts and/or blood loss |
| Intermediate reported cardiac risk (generally <5%) | Carotid endarterectomy |
|
|
Head and neck procedures |
|
|
Intraperitoneal and intrathoracic procedures |
|
|
Orthopedic surgery |
|
|
Prostate surgery |
| Low reported cardiac risk † (generally <1%) | Endoscopic procedures |
|
|
Superficial procedures |
|
|
Cataract surgery |
|
|
Breast surgery |
| From Eagle KA, Berger PB, et al: ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542–553, 2002, with permission. | |
 Figure 27-13a
Coronary risk assessment. Risk stratification strategies
for determining a patient's candidacy for preoperative noninvasive testing before
elective surgery. CHF, congestive heart failure; ECG, electrocardiogram; MET, metabolic
equivalent; MI, myocardial infarction. (Redrawn from Eagle KA, Berger PB,
et al: ACC/AHA guideline update for perioperative cardiovascular evaluation for
noncardiac surgery—executive summary: A report of the American College of
Cardiology/American Heart Association Task Force on Practice Guidelines [Committee
to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac
Surgery]. J Am Coll Cardiol 39:542–553, 2002.)
Figure 27-13a
Coronary risk assessment. Risk stratification strategies
for determining a patient's candidacy for preoperative noninvasive testing before
elective surgery. CHF, congestive heart failure; ECG, electrocardiogram; MET, metabolic
equivalent; MI, myocardial infarction. (Redrawn from Eagle KA, Berger PB,
et al: ACC/AHA guideline update for perioperative cardiovascular evaluation for
noncardiac surgery—executive summary: A report of the American College of
Cardiology/American Heart Association Task Force on Practice Guidelines [Committee
to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac
Surgery]. J Am Coll Cardiol 39:542–553, 2002.)
Since publication of the algorithm in 1996, several studies have suggested that this stepwise approach to the assessment of coronary artery disease is both efficacious and cost-effective.[402] [403] Licker and colleagues compared the data of two consecutive 4-year periods (1993 to 1996 [control period] versus 1997 to 2000 [intervention period]). Implementation of the American College of Cardiology/American Heart Association guidelines[10] was associated with increased use of preoperative stress myocardial imaging (44.3% versus 20.6%; P < .05) and coronary revascularization (7.7% versus 0.8%; P < 0.05). During the intervention period, there was a significant decrease in the incidence of cardiac complications (from 11.3% to 4.5%) and an increase in event-free survival at 1 year after surgery (from 91.3% to 98.2%). Forehlich and colleagues[402] compared 102 historical control patients with 94 patients after implementation of the guideline and 104 patients late after implementation of the guideline. Both resource utilization and cost were reduced after implementation of the guideline, and the effect was sustained for 2 years.
Thus, the major signposts of perioperative myocardial function that we can obtain before surgery are the risk factors for arterial aging and a history of ischemic pain (and its relationship to exercise), CHF, diabetes, and renal insufficiency. The value of this information is slightly enhanced by a 6-minute walk test; determination of the ankle-brachial pressure index; ECG and non-ECG responses to bicycle or treadmill exercise; determination of the ejection fraction; and the use of ECG, radionuclide imaging, radionuclide imaging after dipyridamole, wall motion changes during dobutamine infusion, or angiography.[307] [315] [316] [317] [353] [374] [375] [376] [383] [384] [385] [386] [387] [391] [392] [393] [394] [395] [396] [397] [398] [399] [400] [402] [403] [404]
Much of the information on which altered perioperative anesthetic management of ischemic heart disease is based is derived from studies of patients undergoing aortic and CABG procedures. Although CABG relieves angina and increases exercise tolerance (as did many placebo operations and medications before it),[405] improved survival occurs only in patients with significant left main coronary artery disease[386] [406] and those with mild to moderate impairment in left ventricular function.[407] However, a potential additional benefit of CABG surgery or percutaneous transluminal coronary angioplasty (PTCA) with or without stents has been documented. Reduced perioperative morbidity during subsequent noncardiac surgical procedures may be an additional benefit of surviving CABG surgery or PTCA.[10] [337] [338] [389] [407] [408] [409] Eagle and colleagues reported on a long-term analysis of patients entered into the Coronary Arteries Surgery Study (CASS).[410] They studied patients assigned to medical or surgical treatment of coronary artery disease for over 10 years who subsequently underwent 3368 noncardiac operations in the years after assignment of coronary treatment. The rate of perioperative MI and death was stratified by the type of surgical procedure. Specifically, low-risk surgeries such as skin, breast, urologic, and minor orthopedic procedures were associated with a total morbidity and mortality of less than 1% regardless of coronary treatment type, and previous revascularization did not affect the outcome. Intermediate-risk surgery such as abdominal, thoracic, and carotid endarterectomy was associated with a combined morbidity and mortality of 1% to 5%, with a small but significant improvement in outcome noted in patients who had previously undergone revascularization. The most significant improvement in outcome was seen in patients undergoing major vascular surgery such as abdominal or lower extremity revascularization. In this cohort, mortality after noncardiac surgery was reduced by two thirds in patients who had received bypasses or had revascularization with PTCA (with or without stents). However, this observational study did not randomize patients and was undertaken in the 1970s and 1980s, before significant advances in medical, surgical, and percutaneous coronary strategies.
To provide definitive data for this hypothesis, a randomized controlled study would be necessary; such a trial is currently ongoing.[411] Importantly, CABG surgery may simply constitute a "survival test." That is, it may cause reinfarction or death, or both, in patients who would have sustained an MI or who would have died after noncardiac surgery.[389] This conclusion appears to be likely because patients who do poorly during and after CABG surgery are those with poor left ventricular function and increased left ventricular end-diastolic pressure.[409] Patients with these same cardiovascular conditions also have increased perioperative risk after noncardiac surgery.[315] [317] [337] [338] [367] [374] [375] [376] [383] [384] [385] [386] [387] [390] [392] Therefore, one proposal to decrease perioperative risk in patients severely disabled with angina (or ischemic heart disease) is to study the coronary arteries and perform PTCA (with or without stenting) or CABG, if indicated, before their noncardiac surgery.
Hertzer and associates[337] [338] did just this. Knowing that survival after vascular surgery depends mainly on preserving myocardial function, these investigators obtained coronary angiograms and proposed CABG surgery (when appropriate) for 1001 consecutive patients needing peripheral vascular surgery (regardless of the degree of suspicion of coronary artery disease before angiography). CABG was believed to be indicated in 251 patients, 226 of whom underwent CABG, with 12 (5.31%) operative deaths; of these patients, 130 subsequently underwent peripheral vascular procedures, with only 1 death.
Do these figures imply that mortality was decreased or increased by the CABG procedure? Did some patients not undergo their initially indicated vascular procedure because of the morbidity associated with CABG? (The report did not indicate why 26 patients who initially were scheduled for peripheral vascular procedures did not undergo these procedures after CABG.) The long-term prognosis for Hertzer's patients was better in those who underwent CABG before their noncardiac surgery than in those who did not: the 5-year cardiac survival rate was 82.5% versus 74%. In fact, in the CASS series, previous CABG surgery or PTCA (versus medical therapy) reduced the risk to that of someone 4 years younger ( Fig. 27-14 ).
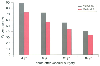 Figure 27-14
Long-term survival of 1834 patients with peripheral vascular
disease who were enrolled in the Coronary Artery Surgery Study (CASS) registry.
Survival rates are shown for those undergoing coronary artery bypass grafting (CABG)
versus medical therapy before their vascular surgery. (Redrawn from Rihal
CS, Eagle KA, Mickel MC, et al: Surgical therapy for coronary artery disease among
patients with combined coronary artery disease and peripheral vascular disease.
Circulation 91:46, 1995.)
Figure 27-14
Long-term survival of 1834 patients with peripheral vascular
disease who were enrolled in the Coronary Artery Surgery Study (CASS) registry.
Survival rates are shown for those undergoing coronary artery bypass grafting (CABG)
versus medical therapy before their vascular surgery. (Redrawn from Rihal
CS, Eagle KA, Mickel MC, et al: Surgical therapy for coronary artery disease among
patients with combined coronary artery disease and peripheral vascular disease.
Circulation 91:46, 1995.)
Are these therapies additive? We do not have an answer. Again, the work of Hertzer and associates describes a nonrandomized series.[337] [338] Huber and colleagues[409] try to make the point that vascular surgery after PTCA is safer than vascular surgery without PTCA, but their series had a rate of serious complications (failed PTCA, MI, death) from PTCA of more than 10%. Posner and coworkers used an administrative data set of patients who underwent PTCA and noncardiac surgery in Washington state. [413] They matched coronary disease patients undergoing noncardiac surgery with and without previous percutaneous coronary interventions (PCI) and looked at cardiac complications. In this nonrandomized design, they noted a significantly lower rate of 30-day cardiac complications in patients who underwent PTCA at least 90 days before the noncardiac surgery. Importantly, PCI within 90 days of noncardiac surgery did not improve outcome. Although the explanation for these results is unknown, they may support the notion that PTCA performed "to get the patient through surgery" may not improve their perioperative outcome because cardiac complications may not occur in patients with stable or asymptomatic coronary stenosis and because PTCA (before embedding drugs into stents or radiation therapy) may actually destabilize some coronary plaques. Patients with destabilized plaques are subject to morbidity early in the increased coagulant phase of the hours, days, or weeks after noncardiac surgery.
PTCA with coronary stenting poses several special issues. Kaluza and coauthors reported on the outcome of 40 patients who underwent prophylactic coronary stent placement less than 6 weeks before major noncardiac surgery requiring general anesthesia.[414] The time between stenting and surgery appeared to be the main determinant of outcome, and the authors recommend that a minimum of 2 weeks and preferably 4 weeks elapse before elective surgery. Wilson and colleagues reported on 207 patients who underwent noncardiac surgery within 2 months of stent placement.[6] A total of 8 patients died or suffered an MI, all of whom were among the 168 patients undergoing surgery within 6 weeks after stent placement. No events occurred in the 39 patients who underwent surgery 7 to 9 weeks after stent placement. These authors suggest that whenever possible, noncardiac surgery should be delayed more than 6 weeks after stent placement, by which time stents are generally endothelialized and a course of antiplatelet therapy to prevent stent thrombosis has been completed.
Therefore, the hypothesis that CABG or PTCA decreases morbidity and mortality in patients undergoing subsequent noncardiac surgery remains a hypothesis. (Although previous CABG or PTCA reduces the risk of subsequent noncardiac surgery, does the combination of revascularization and noncardiac surgery lead to a better outcome than just the noncardiac surgery alone?) In the absence of randomized data, decision analysis models can be used to address the issue.[415] [416] [417] Such models assume that patients with significant coronary artery disease would undergo CABG surgery before noncardiac surgery. The models found that the optimal decision was sensitive to local morbidity and mortality rates within the clinically observed range. These models suggest that preoperative testing for the purpose of coronary revascularization is not the optimal strategy if perioperative morbidity and mortality are low. If long-term survival is included in the models, coronary revascularization may lead to an improved overall outcome and be a cost-effective intervention,[417] particularly in patients with significant left main or three-vessel coronary stenosis (or both). Landesberg and colleagues demonstrated that long-term survival after major vascular surgery is significantly improved if patients with moderate to severe ischemia on preoperative dipyridamole-thallium imaging undergo selective coronary revascularization.[418]
Summarizing a large number of studies, we list the following preoperative findings as conditions that correlate with perioperative morbidity and that can be corrected before surgery:
| NYHA | CCS |
|---|---|
| I. Ordinary physical activity such as walking or climbing stairs does not cause angina. Angina with strenuous or rapid prolonged exertion at work or recreation or with sexual relations | I. Ordinary physical activity such as walking and climbing stairs does not cause angina. Angina with strenuous or rapid or prolonged exertion at work or recreation |
| II. Slight limitation of ordinary activity. Walking or climbing stairs rapidly, walking uphill, and walking or stair climbing after meals or in the cold, in wind, under emotional stress, or only for a few hours after awakening. Walking more than two blocks on the level or more than one flight of stairs at a normal pace and under normal conditions | II. Slight limitation of ordinary activity. Walking or climbing stairs rapidly, walking uphill, and walking or stair climbing after meals or in cold, in wind, when under emotional stress, or only for a few hours after awakening. Walking more than two blocks on the level and climbing more than one flight of ordinary stairs at a normal pace and under normal conditions |
| III. Marked limitation of ordinary physical activity. Walking one or two blocks on the level and climbing one flight of stairs under normal conditions and at a normal pace "Comfortable at rest" | III. Marked limitation of ordinary physical activity. Walking one to two blocks on the level and climbing more than one flight under normal conditions |
| IV. Inability to carry out any physical activity without discomfort—anginal syndrome may be present at rest | IV. Inability to carry out any physical activity without discomfort—anginal syndrome may be present at rest |
Preoperative factors that correlate with perioperative risk but cannot be altered include (1) old physiologic or chronologic age (perioperative risk increases with age), * (2) significant aortic stenosis,[367] [401] [422] (3) emergency surgery,[190] [313] [361] [367] [374] [375] [376] [390] [401] (4) cardiomegaly, [317] [367] [374] [375] [376] [390] [401] (5) history of CHF, † (6) angina (or a history of angina or ischemia) on ECG,[190] [307] [353] [365] [367] [374] [375] [376] [377] [378] [379] [381] [382] [383] [384] [385] [386] [390] (7) abnormal ST-segment or inverted or flat T waves on ECG,[8] [367] [374] [376] abnormal QRS complex on ECG,[367] and (8) a significant mitral regurgitant murmur.[367] [401]
Significant intraoperative factors that correlate with perioperative risk and that may be avoided or altered are as follows: (1) unnecessary use of vasopressors, [423] [424] (2) unintentional hypotension[316] [328] [366] [367] (this point is controversial, however, because some investigators have found that unintentional hypotension does not correlate with perioperative morbidity[421] [424] ), (3) hypothermia,[425] (4) too low or too high a hematocrit,[426] [427] and (5) lengthy operations.[334] [361] [366] [367]
Significant intraoperative factors that correlate with perioperative morbidity and probably cannot be avoided are (1) emergency surgery and (2) thoracic or intraperitoneal surgery or above-the-knee amputations.[334] [365] [366] [367] [374] [375] [376] [377] [378] [379] [381] [382] [383] [384] [385] [386] [387] [388] [389] [390] [392] [401] [420]
Although the evidence for these factors is fairly substantial, virtually no data are derived from prospective randomized studies indicating that treatment of the aforementioned conditions reduces perioperative risk in patients with ischemic heart disease. Nevertheless, all logic dictates that such treatment does reduce risk. Thus, the goal in giving anesthesia to patients with ischemic heart disease is to achieve the best preoperative condition obtainable by treating conditions that correlate with perioperative risk. The next step is to intraoperatively monitor for conditions that correlate with perioperative risk and, by careful attention to detail, avoid circumstances that lead to perioperative risk. Although local anesthesia may reduce perioperative risk,[365] [380] epidemiologic studies do not indicate any significant differences in perioperative morbidity in patients with ischemic heart disease who are given local anesthesia as opposed to general anesthesia.
Preoperative evaluation of a patient with ischemic heart disease should include a review of the clinical course of any previous MIs and a review of studies made subsequent to those events. Because patients most likely to benefit are those who have severe coronary artery disease (i.e., multivessel left main stem coronary artery disease) and ejection fractions of 21% to 50%, some strategies have been devised to limit routine exercise testing, dipyridamole-thallium scanning, Holter monitoring, dobutamine stress echocardiography, and angiographic testing to only these patients ( Fig. 27-15 , Fig. 27-16 , and Fig. 27-17 ).[10] [393] [410] [414] [415] [416] [428] That these studies have been or are being performed implies something about the patient's cardiac function.
The preoperative evaluation should also include a review of the results of exercise studies, Holter monitoring, other noninvasive tests such as doubtamine stress echocardiography, and coronary angiography to determine which ECG lead to monitor for ischemia. Although in theory, the ECG lead that first indicates ischemia or best represents the stenosed artery on exercise should be the first to reveal ischemia in the operating room, no study has confirmed this assumption. If no exercise or coronary angiographic study has been performed, precordial lead V5 is preferred.[8] [307] [429] [430]
The only known way to increase oxygen supply to the myocardium of patients with coronary artery stenosis
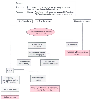 Figure 27-15
Assessment of cardiac risk in a patient about to undergo
vascular surgery. If the clinical index is high, coronary arteriography (CATH) is
recommended, with subsequent coronary artery bypass grafting (CABG) or percutaneous
transluminal coronary angioplasty (PTCA) for those with correctable lesions and patient
(PT) consent. An equivocal history is followed by Holter monitoring and, if Holter
monitoring is abnormal, by dobutamine stress echocardiography. CHF, congestive heart
failure; ICU, intensive care unit; MI, myocardial infarction. (See the text and
data of Fig. 27-9
for analyses
by Mantha and colleagues.[387]
)
Figure 27-15
Assessment of cardiac risk in a patient about to undergo
vascular surgery. If the clinical index is high, coronary arteriography (CATH) is
recommended, with subsequent coronary artery bypass grafting (CABG) or percutaneous
transluminal coronary angioplasty (PTCA) for those with correctable lesions and patient
(PT) consent. An equivocal history is followed by Holter monitoring and, if Holter
monitoring is abnormal, by dobutamine stress echocardiography. CHF, congestive heart
failure; ICU, intensive care unit; MI, myocardial infarction. (See the text and
data of Fig. 27-9
for analyses
by Mantha and colleagues.[387]
)
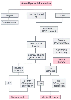 Figure 27-16
Strategy for identifying patients who should undergo
cardiac catheterization after acute myocardial infarction (MI). This strategy is
based on clinical assessment, evaluation of left ventricular (LV) function by radionuclide
angiography (RNA) or echocardiography (Echo), analysis of arrhythmias, and stress
testing. CHF, overt congestive heart failure; ECG, electrocardiogram; EF, ejection
fraction. (Redrawn from Epstein SE, Palmeri ST, Patterson RE: Evaluation
of patients after acute myocardial infarction. Indications for cardiac catheterization
and surgical intervention. N Engl J Med 307:1487, 1982.)
Figure 27-16
Strategy for identifying patients who should undergo
cardiac catheterization after acute myocardial infarction (MI). This strategy is
based on clinical assessment, evaluation of left ventricular (LV) function by radionuclide
angiography (RNA) or echocardiography (Echo), analysis of arrhythmias, and stress
testing. CHF, overt congestive heart failure; ECG, electrocardiogram; EF, ejection
fraction. (Redrawn from Epstein SE, Palmeri ST, Patterson RE: Evaluation
of patients after acute myocardial infarction. Indications for cardiac catheterization
and surgical intervention. N Engl J Med 307:1487, 1982.)
|
|
|
|
|
|
|
|
|
|
|
|
|