|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This section makes nine major points regarding diabetes:
Non-insulin-dependent (type 2) diabetics account for more than 90% of the over 18 million diabetics in the United States,[28] but a diagnosis has been made in only 60%. The medical cost for these patients was over $150 billion in 2002 and has increased at more than 10% per year since 1996. At the current rate of growth, there will be 250 million diabetics in the world in 2010. These individuals tend to be elderly, overweight, and relatively resistant to ketoacidosis and susceptible to the development of a hyperglycemic-hyperosmolar nonketotic state. The diagnosis of diabetes is made with a fasting blood glucose level greater than 125 mg/dL (7.0 mmol/L), and impaired glucose tolerance is diagnosed if the fasting level is below 125 mg/dL (7.0 mmol/L) but greater than 110 (6.1). Plasma insulin levels are normal or elevated in type 2 diabetics but are relatively low for the level of blood glucose. This hyperinsulinemia by itself is postulated to cause accelerated cardiovascular disease.[29]
Insulin originates in the pancreas. Pancreatic islets are composed of at least three cells: alpha cells, which secrete glucagon; beta cells, which secrete insulin; and delta cells, which contain secretory granules. Insulin is first synthesized as proinsulin, converted to insulin by proteolytic cleavage, and then packaged into granules within the beta cells. A large quantity of insulin, normally about 200 U, is stored in the pancreas; continued synthesis is stimulated by glucose. A basal, steady-state release of insulin is provided by the beta granules, in addition to release controlled by stimuli external to the beta cell. Basal insulin secretion continues in the fasted state and is crucial to inhibition of catabolism and ketoacidosis. Glucose and fructose are the primary regulators of insulin release. Other stimulators of insulin release include amino acids, glucagon, GI hormones (gastrin, secretin, cholecystokinin-pancreozymin, and enteroglucagon), and acetylcholine. Epinephrine and norepinephrine inhibit insulin release by stimulating α-adrenergic receptors and stimulate insulin release at β-adrenergic receptors.
Diabetes mellitus is a heterogeneous group of disorders that have the common feature of a relative or absolute lack of insulin. The disease is characterized by a multitude of hormone-induced metabolic abnormalities, by a diffuse microvascular lesion, and by long-term end-organ complications. Diabetes can be divided into two very different diseases that share these end-organ abnormalities. Type 1 diabetes is associated with autoimmune diseases and has a concordance rate of 40% to 50% (i.e., if one of a pair of monozygotic twins had diabetes, the likelihood that the other twin would have diabetes is 40% to 50%). In type 1 diabetes, the patient is insulin deficient and susceptible to ketoacidosis if insulin is withheld. In type 2, the concordance rate is 100% (i.e., genetic material is both necessary and sufficient for the development of type 2 diabetes). These patients are not susceptible to the development of ketoacidosis in the absence of insulin, and they have peripheral insulin resistance. Gestational diabetes develops in more than 2% of all pregnancies and increases the risk for type 2 diabetes to 17% to 63% within 15 years.
Type 1 and type 2 diabetes differ in other ways as well. Contrary to a long-standing belief, patient age does not allow a firm distinction between type 1 and type 2 diabetes; type 1 diabetes can develop in an older person. A diabetes-producing variant of Coxsackie B4 virus has been isolated from the pancreas of a patient who died of diabetic ketoacidosis (type 1 diabetes). This virus was also recovered from mice bred to be diabetes prone after inoculation of the virus had produced hyperglycemia and pancreatic beta cell necrosis coincident with rising
Currently, therapy for type 2 diabetes usually begins with exercise and dietary management. A diet rich in fiber with less saturated fat and daily physical activity for 30 minutes are often associated with normalization of fasting blood glucose levels and delay of glucose intolerance in more than 50% of subjects.[31] The next stage of therapy is the use of oral hypoglycemic medications to stimulate the release of insulin by pancreatic beta cells and improve tissue responsiveness to insulin by reversing the postbinding abnormality. The common orally administered drugs are tolazamide (Tolinase), tolbutamide (Orinase), and the newer sulfonylureas glyburide (Micronase), glipizide (Glucotrol), and glimepiride. These last drugs have a longer blood glucose-lowering effect that persists for 24 hours or more and fewer drug-drug interactions. Oral hypoglycemic drugs may produce hypoglycemia for as long as 50 hours after intake (chlorpropamide [Diabinese] has the longest half-life). Other drugs include metformin, which decreases hepatic glucose output and may increase peripheral responsiveness to glucose (and is associated with lactic acidosis if the patient becomes dehydrated); acarbose, which decreases glucose absorption; and the thiazolidinediones (rosiglitazone and pioglitazone), which increase peripheral responsiveness to insulin.[32] Troglitazone, another drug of this latter class, has been taken off the market because of 61 cases of acute renal failure after its use. Increasingly, physicians advocating tight control of blood sugar levels are giving insulin to "maturity-onset" insulin-dependent diabetic patients twice a day or even more frequently.[33] [34]
Insulin-dependent diabetics tend to be young, nonobese, and susceptible to the development of ketoacidosis. Plasma insulin levels are low or nonmeasurable, and therapy requires insulin replacement. Patients with insulin-dependent diabetes experience an increase in their insulin requirements in the postmidnight hours, which may result in early-morning hyperglycemia (dawn phenomenon). This accelerated glucose production and impaired glucose utilization are due to nocturnal surges in secretion of growth hormone (GH).[35] Normal patients and diabetics taking insulin have steady-state levels of insulin in their blood. (Unfortunately, traditional values for insulin pharmacokinetics are derived from studies designed as though the diabetic received only one shot of insulin in a lifetime.) Absorption of insulin is highly variable and dependent on the type and species of insulin, the site of administration, and subcutaneous blood flow. Nevertheless, attainment of a steady state is dependent on periodic administration of the preparations received by the patient. Thus, it seems logical to perioperatively continue the combinations of preparations that the patient had been receiving chronically after examining the patient's blood glucose monitoring logbook for the degree of control. (In our clinical experience, erratic control can foreshadow perioperative hypoglycemia.)
Acute complications in diabetic patients include hypoglycemia and diabetic ketoacidosis, as well as hyperglycemic, hyperosmolar, nonketotic coma. Diabetic patients are also subject to a series of long-term complications that lead to considerable morbidity and premature mortality, including cataracts, neuropathies, retinopathy, and angiopathy involving peripheral and myocardial vessels. The leading cause of the blindness associated with renal failure in the United States is diabetes. Many of these complications will bring the diabetic patient to surgery. The evidence that hyperglycemia itself accelerates these complications or that tight control of blood sugar levels decreases the rapidity of the progression of microangiopathic disease is becoming more definitive.[17] [18] [36] [37]
Glucose itself may be toxic because high levels can promote nonenzymatic glycosylation reactions leading to the formation of abnormal proteins that may decrease elastance and wound-healing tensile strength.[38] The decrease in elastance is responsible for the stiff joint syndrome and for the fixation of the atlanto-occipital joint that makes intubation difficult.[21] [39] [40] Furthermore, elevations in glucose may increase the production of macroglobulins by the liver, thereby increasing blood viscosity, and may promote intracellular swelling by favoring the production of nondiffusible, large molecules (e.g., sorbitol). Newer drug therapies (e.g., aldose reductase inhibitors) aim to decrease intracellular swelling by inhibiting the formation of such large molecules.
Studies on the long-term outcome of type 1 and type 2 diabetics indicate that tight control of blood glucose and blood sugar substantially prevents such complications ( Table 27-2 ). [17] [18]
Perioperative management of a diabetic patient may affect the surgical outcome. Physicians advocating tight control of blood glucose levels point to the evidence of increased wound-healing tensile strength and decreased wound infections in animal models of diabetes (type 1) under tight control and the development of infections, renal failure, other serious complications, and death in patients requiring intensive care.[14] [15] [16] [41]
Infections account for two thirds of postoperative complications and about 20% of perioperative deaths in diabetic patients. Experimental data suggest that many factors can make a diabetic patient vulnerable to infection. Many alterations in leukocyte function have been demonstrated in hyperglycemic diabetics, including decreased chemotaxis and impaired phagocytic activity of granulocytes, as well as reduced intracellular killing of pneumococci and staphylococci.[42] [43] When diabetic patients are treated aggressively and blood glucose levels are kept below 270 mg/dL, the phagocytic function of granulocytes is improved and intracellular killing of bacteria is restored to nearly normal levels. [44]
Diabetic patients have been thought to experience more infections in clean wounds than nondiabetics do. In a review of 23,649 surgical patients, the rate of wound infection in clean incisions was 10.7% for diabetics versus 1.8% for nondiabetics.[45] However, when age is accounted for, the incidence of wound infection in diabetic surgical patients does not differ significantly from that in nondiabetic patients. In addition, hyperglycemia may worsen the neurologic outcome after intraoperative cerebral ischemia.
|
|
Type 1 Diabetic Patients | |
|---|---|---|
| Measure and Results | Group Undergoing Intensive Control of Blood Glucose | Control Group |
| Measurement of blood glucose levels | ≥4/day | 1/day |
| Insulin injections | ≥3/day, or an insulin pum, was used | 1–2/day |
| Diet | Special diet | Usual diabetic diet |
| Physician office visits | 1–4/mo | Once every 3 mo |
| Results after 6 yr |
|
|
| Diabetic retinopathy | 70% less than control |
|
| Treatment needed for retinopathy | 50% less than control |
|
| Significant nephropathy | 50% less than control |
|
| Neuropathy | 60% less than control |
|
| Modified from Reichard P, Nilsson BY, Rosenqvist U: The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med 329:304–309, 1993. | ||
Blood glucose levels may affect neurologic recovery after a global ischemic event. In a study of 430 consecutive patients resuscitated after out-of-hospital cardiac arrest, mean blood glucose levels were higher in patients who never awakened (341 ± 13 mg/dL) than in those who did awaken (262 ± 7 mg/dL).[46] Among patients who awakened, those with persistent neurologic deficits had higher mean glucose levels (286 ± 15 mg/dL) than did patients without deficits (251 ± 7 mg/dL). These results are consistent with the finding that hyperglycemia during a stroke is associated with poorer short- and long-term neurologic outcomes. Although several questions remain, the likelihood that blood glucose is a determinant of brain damage after global ischemia is supported by the vast majority of animal studies after global CNS ischemia[20] and by most, but not all studies after focal CNS ischemia.[20] The preponderance of data after CNS ischemia indicates that levels of glucose higher than 150 or 250 mg/dL have an adverse effect on CNS recovery. Until better data are available, many will argue that a diabetic patient about to undergo surgery in which hypotension or reduced cerebral blood flow may occur should have a blood glucose level below 200 mg/dL during a period of cerebral ischemia. Some special situations may also influence how tightly one should manage a patient's glucose level, such as surgery requiring cardiopulmonary bypass, surgery in pregnant patients, and emergency surgery in patients with diabetic ketoacidosis or hyperosmolar nonketotic coma.
Diabetics undergoing coronary artery bypass graft (CABG) surgery in 1980 had a perioperative mortality rate of 5% versus 1.5% for nondiabetics.[47] In this study and in most other studies of diabetic patients undergoing CABG surgery, important additional risk factors or confounding variables were not considered, including the incidence and extent of hypertension, ventricular dysfunction, and CHF or the severity of coronary artery disease.
The aforementioned study of 340 diabetics and 2522 nondiabetics undergoing CABG surgery in 1980 demonstrated only a moderate increase in operative mortality for diabetics (1.8% versus 0.6%).[47] In the postbypass phase, patients with diabetes were found to require inotropic therapy and intra-aortic balloon pump support five times more frequently than nondiabetics did. This increased need for therapy in diabetics is due to several possible factors. Diabetics with angina have more extensive coronary artery disease than nondiabetic patients do. They are also more likely to have hypertension, cardiomegaly, diffuse hypokinesis, and previous myocardial infarction (MI). Insulin-dependent diabetics with coronary artery disease, impaired stress responses, and autonomic nerve dysfunction appear to have stiffer ventricles with a greater elevation in left ventricular end-diastolic pressure than matched nondiabetic controls do.[48] [49] [50] During cardiopulmonary bypass, hypothermia and stress reactions decrease the response to insulin and result in marked hyperglycemia (even when the perfusate and intravenous solutions do not contain glucose). Administration of washed cells has been advocated for small individuals because acid citrate dextrose- or adenine-supplemented blood can result in significant hyperglycemia. These changes are exaggerated in diabetic patients, and administration of insulin may have little effect until rewarming is achieved. In a number of recently reported cases, inotropic agents were ineffective in maintaining cardiac contractility, although filling pressures, sinus rhythm, serum electrolytes, and arterial blood gases (ABGs) were adequate. Blood sugar levels were elevated in each case. After intravenous infusion of insulin, effective myocardial contractions returned and allowed easy and rapid weaning from bypass. The effect of glucose levels on neurologic outcome during cardiopulmonary bypass is both unclear and controversial.
Many diabetics requiring emergency surgery for trauma or infection have significant metabolic decompensation, including ketoacidosis. Frequently, little time is available for stabilization of the patient, but even a few hours may be sufficient for correction of any fluid and electrolyte disturbances that are potentially life threatening. It is futile to delay surgery in an attempt to eliminate ketoacidosis completely if the underlying surgical condition will lead to further metabolic deterioration. The likelihood of intraoperative cardiac arrhythmias and hypotension resulting from ketoacidosis will be reduced if volume depletion and hypokalemia are at least partially treated.
Insulin therapy is initiated with a 10-U intravenous bolus of regular insulin, followed by continuous insulin infusion. The rate of infusion is determined most easily if one divides the last serum glucose value by 150 (or 100 if the patient is receiving steroids, has an infection, or is considerably overweight). The actual amount of insulin administered is less important than regular monitoring of glucose, potassium, and pH. Because the number of insulinbinding sites is limited, the maximum rate of glucose decline is fairly constant and averages 75 to 100 mg/dL/hr, regardless of the dose of insulin.[51] During the first 1 to 2 hours of fluid resuscitation, the glucose level may fall more precipitously. When serum glucose reaches 250 mg/dL, the intravenous fluid should include 5% dextrose.
The volume of fluid required for therapy varies with the overall deficit; it ranges from 3 to 5 L and may be as high as 10 L. Despite losses of water in excess of losses of solute, sodium levels are generally normal or reduced. Factitious hyponatremia caused by hyperglycemia or hypertriglyceridemia may result in this seeming contradiction. The plasma sodium concentration decreases by about 1.6 mEq/L for every 100-mg/dL increase in plasma glucose above normal. Initially, normal saline is infused at a rate of 250 to 1000 mL/hr, depending on the degree of volume depletion and cardiac status. Some measure of left ventricular volume should be monitored in diabetics who have a history of myocardial dysfunction. About a third of the estimated fluid deficit is corrected during the first 6 to 8 hours and the remaining two thirds over the next 24 hours.
The degree of acidosis is determined by measurement of ABGs and
detection of an increased anion gap:
Na+
− (Cl−
+ HCO3
−
)
Acidosis with an increased anion gap (≥16 mEq/L) in an acutely ill diabetic may
be caused by ketones in ketoacidosis, lactic acid in lactic acidosis, increased organic
acids from renal insufficiency, or all three problems. In ketoacidosis, plasma levels
of acetoacetate, β-hydroxybutyrate, and acetone are increased. Plasma and urinary
ketones are measured semiquantitatively with Ketostix and Acetest tablets. The role
of bicarbonate therapy in diabetic ketoacidosis is controversial. Myocardial function
and respiration are known to be depressed at a blood pH below 7.0 to 7.10, yet rapid
correction of acidosis with bicarbonate therapy may result in alterations in CNS
function and structure. These alterations may be caused by (1) paradoxical development
of cerebrospinal fluid and CNS acidosis from rapid conversion of bicarbonate to carbon
dioxide and diffusion of the acid across the blood-brain barrier, (2) altered CNS
oxygenation with decreased cerebral blood flow, and (3) the development of unfavorable
osmotic gradients. After treatment with fluids and insulin, β-hydroxybutyrate
levels decrease rapidly, whereas acetoacetate levels may remain stable or even increase
before declining. Plasma acetone levels remain elevated for 24 to 42 hours, long
after blood glucose, β-hydroxybutyrate, and acetoacetate levels have returned
to normal; the result is continuing ketonuria.[51]
Persistent ketosis with a serum bicarbonate level less than 20 mEq/L in the presence
of a normal glucose concentration is an indication of the continued need for intracellular
glucose and insulin for reversal of lipolysis.
The most important electrolyte disturbance in diabetic ketoacidosis is depletion of total-body potassium. Deficits range from 3 to 10 mEq/kg body weight. Serum potassium levels decline rapidly and reach a nadir within 2 to 4 hours after the start of intravenous insulin administration. Aggressive replacement therapy is required. The potassium administered moves into the intracellular space with insulin as the acidosis is corrected. Potassium is also excreted in urine because of the increased delivery of sodium to the distal renal tubules that accompanies volume expansion. Phosphorus deficiency in ketoacidosis as a result of tissue catabolism, impaired cellular uptake, and increased urinary losses may give rise to significant muscular weakness and organ dysfunction. The average phosphorus deficit is approximately 1 mmol/kg body weight. Replacement is needed if the plasma concentration falls below 1.0 mg/dL.[51]
Chronic tight control of blood glucose has been motivated by a theoretical concern about five potential glucotoxicities, plus the results from three major randomized outcome studies involving diabetic patients. [17] [18]
Glucose itself may be toxic because high levels can promote nonenzymatic glycosylation reactions that lead to the formation of abnormal proteins. These proteins may decrease elastance, which is responsible for the stiff joint syndrome (and difficult intubation secondary to fixation of the atlanto-occipital joint), as well as decrease wound-healing tensile strength. Furthermore, elevations in glucose may increase macroglobulin production by the liver (which would increase blood viscosity) and promote intracellular swelling by favoring the production of nondiffusible, large molecules (such as sorbitol). Some drug therapies (e.g., aldose reductase inhibitors) endeavor to decrease intracellular swelling by inhibiting the formation of such large molecules.
Glycemia also disrupts autoregulation. Glucose-induced vasodilation prevents target organs from protecting against increases in systemic BP. A glycosylated hemoglobin level of 8.1% is the threshold at which the risk of microalbuminuria increases logarithmically. A person with type 1 diabetes who has microalbuminuria greater than 29 mg/day has an 80% chance of experiencing renal insufficiency. The threshold for glycemic toxicity differs for various vascular beds. For example, the threshold for retinopathy is a glycosylated hemoglobin value of 8.5% to 9.0% (12.5 mmol/L or 225 mg/dL), and that for cardiovascular disease is an average blood glucose value of 5.4 mmol/L (96 mg/dL). Thus, different degrees of hyperglycemia may be required before different vascular beds are damaged, or certain degrees of glycemia are associated with other risk factors for vascular disease. Another view is that perhaps severe hyperglycemia and microalbuminuria are simply concomitant effects of a common underlying cause. For instance, diabetics in whom microalbuminuria develops are more resistant to insulin, insulin resistance is associated with microalbuminuria in first-degree relatives of type 2 diabetics, and persons who are normoglycemic but subsequently have clinical diabetes are at risk for atherogenesis before the onset of disease.
Tight control retards all these glucotoxicities and may have other benefits in retarding the severity of diabetes itself.[17] [18] [52]
Thus, management of intraoperative glucose might be influenced by specific situations, such as the type of operation, pregnancy,[53] [54] and the expected global CNS insult; the bias of the patient's primary care physician; or the type of diabetes. Type 1 diabetic patients definitely need insulin and might be considered candidates for tight control of blood glucose levels. Type 2 diabetic patients have insulin, and current data indicate they do not benefit from tight perioperative control unless they need intensive care.[14] [15] [16] [55]
The key to managing blood glucose levels perioperatively in diabetic patients is to set clear goals and then monitor blood glucose levels frequently enough to adjust therapy to achieve these goals. Three regimens that afford various degrees of perioperative control of blood glucose levels are discussed in the following sections.
Such a regimen has been found to meet its goals.[56]
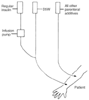 Figure 27-1
Arrangement of intravenous lines for infusion of regular
insulin in a regimen tightly controlling blood glucose levels in diabetic patients
undergoing surgery. D5W, 5% dextrose in water.
Figure 27-1
Arrangement of intravenous lines for infusion of regular
insulin in a regimen tightly controlling blood glucose levels in diabetic patients
undergoing surgery. D5W, 5% dextrose in water.
Although we have not found it necessary to treat hypoglycemia (i.e., blood glucose levels <50 mg/dL), we have been prepared to do so with 15 mL of 50% dextrose in water. In such circumstances, the insulin infusion would be terminated. This regimen has been found to accomplish its objectives, with the exception of such tight goals for blood glucose, even in very "brittle" diabetics (i.e., those extremely resistant to treatment) given high doses of steroids.[58]
This last regimen may well supersede all others if the cost of a mechanical pancreas can be reduced and if control of hyperglycemia is shown to make a meaningful difference perioperatively; it has superseded all others in many ICUs, and for good reason ( Table 27-3 ).[14] [15] [16]
| Glucose Therapy (mg/dL) | Intensive Rx (80–110) | Conventional Rx (180–200) |
|---|---|---|
| Death in the ICU | 4.6% | 8.0% |
| After 5 days in the ICU | 10.6% | 20.2% |
| 1st 5 ICU days | 1.7% | 1.8% |
| All deaths | 7.2% | 10.9% |
| >14 days in the ICU | 11.4% | 15.7% |
| >14 days ventilated | 7.5% | 11.9% |
| Rx dialysis | 4.8% | 8.2% |
| Polyneuropathy | 28.7% | 51.9% |
Adverse perioperative outcomes have repeatedly and substantially correlated with the age of the patient,[7] [9] [12] [26] [27] [59] [60] [61] and diabetes does cause physiologic aging. When one translates the results of the Diabetes Control and Complications Trials into age-induced physiologic changes, a type 1 diabetic who has poor control of blood sugar ages approximately 1.75 years physiologically for every chronologic year of the disease and 1.25 years if blood sugar has been controlled tightly.[26] A type 2 diabetic ages about 1.5 years for every chronologic year of the disease and about 1.06 years with tight control of blood sugar and BP.[18] [26] [27] [31] Thus, when providing care for a diabetic patient, one must consider the associated risks to be those of a person who is much older physiologically. That is, a diabetic's physiologic age ("RealAge") is considerably higher than that person's calendar age just by virtue of having the disease.[1]
The increased prevalence of obesity and lack of physical exercise seem to be major contributors to the increased prevalence of type 2 diabetes. As with type 1 diabetes, tight control of blood sugar, increased physical activity, and reduction in weight appear to reduce the accelerated aging associated with type 2 diabetes and even to substantially delay appearance of the disease. Although such a reduction in aging should reduce the perioperative risk for diabetic patients, no controlled trials have confirmed this theory.
Diabetes is associated with microangiopathy (in retinal and renal vessels), peripheral neuropathy, autonomic dysfunction, and infection. Diabetics are often treated with angiotensin-converting enzyme (ACE) inhibitors, even in the absence of gross hypertension, in an effort to prevent the effects of disordered autoregulation, including renal failure.[17] [18] [62]
Before surgery, assessment and optimization of treatment of the potential and potent end-organ effects of diabetes are at least as important as assessment of the diabetic's current overall metabolic status. Information about a diabetic patient that might merit special attention before surgery includes the therapeutic, dietary, and exercise or physical activity regimens; adequacy of glucose control; previous surgical and anesthetic responses; and presence of the end-organ effects of diabetes. In our experience, many diabetic patients pay extreme attention to glucose control, expect each physician to ask about it, and are annoyed (and probably rightly so) if the physicians treating them in the perioperative period are not at least as concerned about the glucose level as the patients have had to be. Thus, if just to avoid making the patient angry (and, we believe, for more value than that), the anesthesiologist should inquire in some depth about the diabetic's control of blood glucose levels. Basic laboratory examinations might include determination of fasting blood sugar levels, electrolytes, and blood urea nitrogen (BUN) or creatinine levels, as well as an electrocardiogram (ECG). Scheduling the operative procedure early in the day avoids prolonging the catabolic state and minimizes the risk of preoperative hypoglycemia.
If one is to believe the few studies we have, the presence of autonomic neuropathy makes the operative period more hazardous and the postoperative period crucial to survival. Evidence of autonomic neuropathy might be routinely sought before surgery. Patients with diabetic autonomic neuropathy are at increased risk for gastroparesis (and consequent aspiration) and for intraoperative and postoperative cardiorespiratory arrest. Data indicate that diabetics who exhibit signs of autonomic neuropathy, such as early satiety, lack of sweating, lack of pulse rate change with inspiration or orthostatic maneuvers, and impotence, have a very high incidence of painless myocardial ischemia[50] [57] [58] [59] [63] [64] and gastroparesis. Some investigators have successfully used 10 mg of metoclopramide preoperatively to facilitate gastric emptying of solids ( Fig. 27-2 ). Interference with respiration or sinus automaticity by pneumonia or by anesthetics, pain medications, or sedative drugs appears to be the precipitating cause in most cases of sudden cardiorespiratory arrest. Measuring the degree of sinus arrhythmia or beat-to-beat variability provides a simple, accurate test for significant autonomic neuropathy. The difference between the maximum and minimum heart rate on deep inspiration is normally 15 beats/min but was found to be 5 beats/min or less in all patients who sustained cardiorespiratory arrest.[63]
Other characteristics of patients with autonomic neuropathy include postural hypotension with a decrease in BP of more than 30 mm Hg, resting tachycardia, nocturnal diarrhea, and dense peripheral neuropathy. Diabetics with significant autonomic neuropathy may have impaired respiratory responses to hypoxia and are particularly susceptible to the action of drugs that have depressant effects. These patients may warrant very close, continuous cardiac and respiratory monitoring for 24 to 72 hours postoperatively, although such logical treatment has not been tested in a rigorous, controlled trial.[23] In the absence of autonomic neuropathy, we would favor outpatient surgery for a diabetic ( Table 27-4 ).
At least four major changes in care for diabetic patients have made it to the clinical trial stage:
 Figure 27-2
Gastric emptying time (mean ± SD) of a solid test
meal in three groups of patients: diabetics (line 1), diabetics given metoclopramide
(10 mg intravenously) 1.5 hours before the test meal (line 2), and nondiabetics (line
3). (From Wright RA, Clemente R, Wathen R: Diabetic gastroparesis: An
abnormality of gastric emptying of solids. Am J Med Sci 289:240, 1985.)
Figure 27-2
Gastric emptying time (mean ± SD) of a solid test
meal in three groups of patients: diabetics (line 1), diabetics given metoclopramide
(10 mg intravenously) 1.5 hours before the test meal (line 2), and nondiabetics (line
3). (From Wright RA, Clemente R, Wathen R: Diabetic gastroparesis: An
abnormality of gastric emptying of solids. Am J Med Sci 289:240, 1985.)
| Outpatient If | Morning Admittance Patient If |
|---|---|
| Can evaluate history in advance | Cannot evaluate history |
| End-organ disease does not require monitoring | End-organ disease requires invasive monitoring |
| Prehydration is available or is unnecessary | Needs careful prehydration |
| No CNS ischemia or planned cardiopulmonary bypass | CNS ischemia is present or cardiopulmonary bypass is planned |
| Not pregnant | Pregnant |
| Patient or vested home "mate" can determine blood glucose level | Patient cannot determine blood glucose level |
| Has vested home "mate" | No vested individual |
| Can take temperature or look for "red" wound | Cannot take temperature or look for "red" wound |
| Plan higher admit rate (no data) | Social care network is unsuitable |
You can imagine how some of these treatments may radically change the therapies used in the perioperative period. If regrowth of islets becomes common, type 1 diabetes could all but disappear; if implanted minute-to-minute glucose reading is possible, tight control may be much easier and more expected.
Perhaps as important as arranging for tight control in a diabetic needing intensive care is keeping in mind that diabetic patients have an increased incidence of atherosclerosis and all its complications. These patients are particularly susceptible to episodes of painless myocardial ischemia and cardiovascular instability. [65] [66] [67] In fact, over 80% of the ischemic episodes that occur in both patients who have myocardial ischemia and those who are diabetic are "silent." However, a 65-year-old diabetic is as likely to die of a cardiovascular event in the next several years as a person who has just had a heart attack ( Table 27-5 ).[68] These data dramatically illustrate the increased risk of cardiovascular disease in diabetics who do not have symptoms. As with other endocrinopathies, the cardiovascular system should be a focus for the anesthetist's attention in diabetic patients.
Hypoglycemia in persons not treated for diabetes is rare. Hypoglycemia in nondiabetics can be caused by such diverse entities as pancreatic islet cell adenoma or carcinoma, large hepatoma, large sarcoma, alcohol ingestion, use of β-adrenergic receptor blocking drugs, haloperidol, hypopituitarism, adrenal insufficiency, altered physiology after gastric or gastric bypass surgery, hereditary fructose intolerance, antidiabetic drug ingestion, galactosemia, or autoimmune hypoglycemia.[69] The last four entities cause postprandial reactive hypoglycemia. Because restriction of oral intake prevents severe hypoglycemia, the practice of keeping the patient NPO and infusing small amounts of a solution containing 5% dextrose greatly lessens the possibility of perioperative postprandial reactive hypoglycemia. The other causes of hypoglycemia can cause serious problems during the perioperative period.[70]
Symptoms of hypoglycemia fall into two groups: adrenergic excess (tachycardia, palpitations, tremulousness, or diaphoresis) and neuroglycopenia (headache, confusion, mental sluggishness, seizures, or coma). All these symptoms may be masked by anesthesia, so blood glucose levels should be determined frequently in such patients to ensure that hypoglycemia is not present. Because manipulation of an insulinoma can result in massive insulin release, this tumor should probably be operated on only at centers equipped with a mechanical pancreas; new data may indicate that the somatostatin analog octreotide will make such operations much less risky in all settings. [71] [72]
Muir and colleagues[71] managed 38 patients undergoing resection of insulinoma. Every 15 minutes these investigators determined the plasma glucose concentration in these patients, in whom a mechanical pancreas produced no increase in plasma glucose. Although 9 of the 38 patients became significantly hypoglycemic (i.e., plasma glucose concentrations <50 mg/dL), only 4 of 253 measurements taken before resection showed a decrease in glucose of
|
|
Nondiabetics |
|
|
|---|---|---|---|
|
|
Without Previous MI (n = 1304) | With Previous MI (n = 69) | Type 2 Diabetics without Previous MI (n = 890) |
| Fatal or nonfatal MIs, per 100 person-years | 0.5 | 3.0 | 3.2 |
| Death from cardiovascular causes, per 100 person-years | 0.3 | 2.6 | 2.5 |
| Adapted from Haffner SM, Lehto S, Rönnemaa T, et al: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229, 1998, with permission. | |||
Perioperative use of the somatostatin analog octreotide, which suppresses insulin release from such tumors, appears to make the perioperative period a logarithm safer in anecdotal experience. Whether all tumors respond to this drug similarly and thus radically reduce the hazard from resection of insulinoma remains to be determined.[72]
|
|
|
|
|
|
|
|
|
|
|
|
|