|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hyperlipidemia may result from obesity, estrogen or corticoid therapy, uremia, diabetes, hypothyroidism, acromegaly, alcohol ingestion, liver disease, inborn errors of metabolism, or pregnancy. Hyperlipidemia may cause premature coronary or peripheral vascular disease or pancreatitis.[73] Most cholesterol is carried in serum by low-density lipoprotein (LDL), whereas approximately 30% of total serum cholesterol is carried by high-density lipoprotein (HDL). Increased LDL cholesterol, a form of hyperlipidemia, appears to be associated with premature atherosclerosis, as is decreased HDL cholesterol. HDL cholesterol is carried in roughly equivalent amounts on two types of HDL: on a less dense HDL2 subfraction that is negatively associated with coronary artery disease and on a more dense HDL3 subfraction that is unrelated to coronary artery disease.
In regard to the production of atherosclerosis, LDL is distinguished from HDL by associating L with "lousy" and H with "healthy." That is, the oxidized form of LDL constitutes a risk factor for atherosclerosis, whereas HDL is believed to carry dangerous cholesterol away from the periphery, to be metabolized by the liver, and is therefore protective. Levels of HDL are 25% higher in women than men; low levels of HDL in women are associated with premature atherosclerosis. Cigarette smoking lowers HDL levels, whereas regular exercise (particularly strenuous exercise, but even nonstrenuous exercise) and a small daily intake of alcohol raise HDL levels. However, alcohol increases HDL3 , the HDL subfraction thought to be inert with respect to coronary artery disease; octogenarians have high levels of HDL.
Data showing that coronary events can be reduced by treating individuals with even normal levels of LDL cholesterol with the "statins"—drugs that raise HDL and lower LDL cholesterol levels—resulted from a decade of rapid progress in preventing reinfarction in high-risk patients.[74] [75] [76] [77] [78] Secondary prevention efforts were successful when these high-risk patients stopped smoking, reduced their BP, controlled stress, increased physical activity, and used aspirin, folate, β-blocking drugs, angiotensin inhibitors, diet, and other drugs to reduce their levels of LDL and increase their levels of HDL.[79] [80]
Although controlling the diet remains a major treatment modality for all types of hyperlipidemia,[79] [81] the drugs fenofibrate and gemfibrozil, which are used to treat hypertriglyceridemia, can cause myopathy, especially in patients with hepatic or renal disease; clofibrate is also associated with an increased incidence of gallstones. Cholestyramine binds bile acids, as well as oral anticoagulants, digitalis drugs, and thyroid hormones. Nicotinic acid causes peripheral vasodilation and should probably not be continued through the morning of surgery. Probucol (Lorelco) decreases the synthesis of apoprotein A-I; its use is associated on rare occasion with fetid perspiration or prolongation of the QT interval (or both) and sudden death in animals.
The West of Scotland Coronary Prevention Study and its congeners produced convincing evidence that drugs in the "statin" class (3-hydroxy-3-methylglutaryl-coenzyme A [HMG-CoA] reductase inhibitors) prevent the morbidity and mortality related to arterial aging and vascular disease, as well as their consequences, such as coronary arterial disease, stroke, and peripheral vascular insufficiency.[75] [76]
|
|
Lovastatin Group (20–40 mg/day) | Placebo Group | P Value |
|---|---|---|---|
| No. of patients | 3304 | 3301 | NS |
| % men | 85% | 85% | NS |
| Age (mean ± SD, yr) |
|
|
|
| Men | 58 ± 7 | 58 ± 7 | NS |
| Women | 62 ± 5 | 63 ± 5 | NS |
| First acute major coronary events | 116 | 183 | <.001 |
| Unstable angina | 60 | 87 | .02 |
| Myocardial infarction | 57 | 95 | .002 |
| Revascularization | 106 | 157 | .001 |
| Coronary events | 163 | 215 | .006 |
| Abstracted from Downs JR, Clearfield M, Weis S, et al: Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 279:1615, 1998, with permission. | |||
However, the report of Downs and coworkers[75] from the Air Force/Texas Coronary Atherosclerosis Prevention Study went further. It showed a 37% reduction in the risk of first acute major coronary events in patients who had not only no risk factors for coronary artery aging but also normal (average) LDL cholesterol levels ( Table 27-6 ). In this study lovastatin did not alter mortality rates, but that had been true for many early short-term trials with the statins. Although much of the effect of the statins has been attributed to their lipid-lowering effects, statins have also been shown to modify endothelial function, inflammatory responses, plaque stability, and thrombogenicity.[80] The report of Downs and colleagues broadened the use of statins, and they remain mainstays of therapy for hyperlipidemia. Statins are drugs that block HMG-CoA reductase, the rate-limiting enzyme of cholesterol synthesis. Their use is occasionally accompanied by liver dysfunction, CNS dysfunction, and severe depression not related to the high cost of each drug and its cogeners. Recent data indicate that statins provide the substantial benefit of reversing inflammation in arteries, as evidenced by their ability to decrease highly specific C-reactive protein (hs-CRP) and pull cholesterol from plaques.[79] [82] [83]
Hypolipidemic conditions are rare diseases often associated with neuropathy, anemia, and renal failure. Although anesthetic experience with hypolipidemic conditions has been limited, some specific recommendations can be made: continuation of caloric intake and intravenous administration of protein hydrolysates and glucose throughout the perioperative period.
No randomized controlled trials give irrefutable evidence that preoperative evaluation of obese patients, even before bariatric surgery, makes a significant difference in patient mortality. However, much evidence indicates that such evaluations, as for other patients, makes the perioperative period more efficient, reduces provider and patient anxiety, and sets appropriate expectations that lead to increased patient satisfaction with pain therapy and the entire perioperative experience. We believe that such an assessment can also spot important variations in airway, pulmonary, cardiovascular, metabolic, and nervous system physiology that lead to improved provider preparedness, patient outcome, and the feeling of both about the quality of the perioperative care. Such may be an important element of surgery in the obese, especially for bariatric surgery. Moreover, motivating and ensuring prophylaxis for deep vein thrombosis can be an important determinant of the acute outcome inasmuch as pulmonary emboli are the greatest cause of perioperative 30-day mortality. Importantly, any contribution that the anesthesiologist can make toward evaluating the psychological attitude and preparation of the patient (for example, the patient stopped exercising 3 weeks ago) is usually most welcomed and important to the surgeon. For it is the psychological readiness of each patient and that patient's commitment to outcome after this operation that appear to determine its long-term worth for that patient.
Obesity in society and in an individual may not be of recent origin, but the rapid increase in treatments involving successful surgery for obesity without huge morbidity is. Recently, the rate and number of obese persons in all societies have been increasing with epidemic speed. In fact, obesity may be increasing even faster than surgery for it is. For example, in the United States, more than 50% of adults weigh more than 20% above what is considered the optimum body weight for their height—a recent increase from 30%.[84] Furthermore, this increase occurred in just 18 years. One measure of obesity is body mass index (BMI), for which a value above 31 kg/m2 represents morbid obesity and its risks and a value above 27 for women and 28 for men corresponds to weight 25% above ideal ( Table 27-7 ). The pathophysiologic consequences of obesity involve every major organ system.[85] Many of the metabolic, hormonal, and physiologic changes associated with obesity (e.g., insulin resistance, decreased number of
|
|
Average Weight (Men and Women) † |
|
||||||
|---|---|---|---|---|---|---|---|---|
| Height ‡ | 19–34 yr | ≥35 yr |
|
|||||
| ft/in | cm | lb | Average kg | Average BMI | lb | Average kg | Average BMI | BMI 20% 〉 Ideal |
| 5'0" | 152.4 | 97–128 | 51.1 | 22.0 | 108–138 | 55.9 | 24.1 | 27.5 |
| 5'1" | 154.9 | 101–132 | 53.0 | 22.0 | 111–143 | 57.7 | 24.8 | 27.5 |
| 5'2" | 157.5 | 104–137 | 54.7 | 22.0 | 115–148 | 59.5 | 23.9 | 27.5 |
| 5'3" | 160.0 | 107–141 | 56.4 | 22.0 | 119–152 | 61.5 | 24.0 | 27.5 |
| 5'4" | 162.6 | 111–146 | 58.4 | 22.0 | 122–157 | 63.4 | 24.0 | 27.5 |
| 5'5" | 165.1 | 114–150 | 60.0 | 22.0 | 126–162 | 65.5 | 24.0 | 27.5 |
| 5'6" | 167.6 | 118–155 | 62.0 | 22.0 | 130–167 | 67.5 | 24.0 | 27.5 |
| 5'7" | 170.2 | 121–160 | 63.9 | 22.0 | 134–172 | 69.5 | 24.0 | 27.5 |
| 5'8" | 172.7 | 125–164 | 65.7 | 22.0 | 138–178 | 71.5 | 24.0 | 27.5 |
| 5'9" | 175.3 | 129–169 | 67.7 | 22.0 | 142–183 | 73.8 | 24.0 | 27.5 |
| 5'10" | 177.8 | 132–174 | 69.6 | 22.0 | 146–188 | 75.9 | 24.0 | 27.5 |
| 5'11" | 180.3 | 136–179 | 71.6 | 22.0 | 151–194 | 78.4 | 24.1 | 27.5 |
| 6'0" | 182.9 | 140–184 | 73.7 | 22.0 | 155–199 | 80.4 | 24.0 | 27.5 |
| 6'1" | 185.4 | 144–189 | 75.7 | 22.0 | 159–205 | 82.7 | 24.0 | 27.5 |
| 6'2" | 188.0 | 148–195 | 77.9 | 22.0 | 164–210 | 85.0 | 24.0 | 27.5 |
| 6'3" | 190.5 | 152–200 | 80.0 | 22.0 | 168–216 | 87.2 | 24.0 | 27.5 |
| Data from Roizen MF: Preoperative Test Selection Program Software Manual of Version 2.15L. Pleasanton, CA, Nellcor, 1994. | ||||||||
Although many conditions associated with obesity (diabetes, hyperlipidemia, cholelithiasis, gastroesophageal reflux disease, cirrhosis, degenerative joint and disk disease, venous stasis and thrombotic/embolic disease, sleep disorders, and emotional and altered body image disorders) contribute to chronic morbidity in the obese, the main concerns for the anesthesiologist have been the same for over 3 decades—derangements of the cardiopulmonary system.[88] [89]
In obese patients, gas exchange may be impaired not only by altered cardiopulmonary mechanisms but also by management of a difficult airway, loss of functional residual capacity (FRC), and ensuing rapid desaturation when anesthesia is induced; by a propensity for desaturation in the recumbent (as opposed to the upright) position and the potential need to induce anesthesia and recovery in such patients in the upright position; by a propensity for and the presence of sleep apnea, chronic respiratory insufficiency, pulmonary hypertension, and a predisposition to deep vein thrombosis and its consequences; and by a need for active participation to overcome uncomfortableness and motivate ambulation in the postoperative period.
Obstruction of the airway by the abundant soft tissue in the upper airway frequently produces hypoxemia and hypercapnia, and obesity significantly increases the risk of difficult tracheal intubation.[90] [91] FRC decreases because the weight of the torso and abdomen makes diaphragmatic excursions more difficult and more position dependent, a problem made worse by mechanical ventilation. For many obese patients, this process causes FRC to be less than closing volume and decreases the time of safe apnea before hypoxia occurs (Amalraj S, personal communication) ( Fig. 27-3 ). Furthermore, obese patients have increased volume and acidity of gastric juices preoperatively ( Fig. 27-4 ), perhaps indicating the wisdom of premedicating such patients with cimetidine, ranitidine, Bicitra, or metoclopramide. (The simplest, least expensive, scientifically tested regimen appears to be administration of cimetidine the night and morning before surgery [ Fig. 27-5 ] [Amalraj S, personal communication]). Others would conclude that awake intubation is indicated, but a preference for this technique must be balanced by the realization that most episodes of gastroesophageal reflux and the greatest
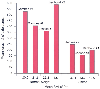 Figure 27-3
Duration of safe apnea before hemoglobin desaturation
in obese and normal-weight patients (90% oxygen saturation), as reported by various
studies. BMI, body mass index. (Amalraj S, personal communication.)
Figure 27-3
Duration of safe apnea before hemoglobin desaturation
in obese and normal-weight patients (90% oxygen saturation), as reported by various
studies. BMI, body mass index. (Amalraj S, personal communication.)
Morbid obesity (BMI >30 kg/m2 ) is the most common and a major risk factor for obstructive sleep apnea (OSA).[96] An increase in BMI by about two units (e.g., from 27 to 29) increases the likelihood of coexisting OSA by a factor of 4.[97] OSA in the obese generally improves after weight reduction.[98] Whereas the prevalence of OSA in the
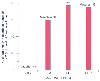 Figure 27-4
Relationship between weight and risk factors for pulmonary
aspiration, as reported by four studies. (Amalraj S, personal communication.)
Figure 27-4
Relationship between weight and risk factors for pulmonary
aspiration, as reported by four studies. (Amalraj S, personal communication.)
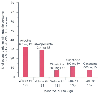 Figure 27-5
Effect of drugs on the risk of pulmonary aspiration in
obese patients, as reported by various studies. (Amalraj S, personal communication.)
Figure 27-5
Effect of drugs on the risk of pulmonary aspiration in
obese patients, as reported by various studies. (Amalraj S, personal communication.)
The presence and severity of OSA in obese patients cannot be reliably predicted by BMI, neck circumference, pulmonary function tests, daytime room-air ABGs, or questionnaires to detect and quantify sleep-related complaints (or any combination of these factors).[102] [103] [104] [105] [106] In the United States, sleep apnea is undiagnosed in 80% to 90% of sufferers.[97] [107] Consequently, preoperative patients with this condition frequently have suggestive symptoms, but no previous evaluation. (Bariatric surgery may be a better option than chronic tracheostomy in patients with OSA, and the need for tracheostomy is frequently listed as an indication for such bariatric surgery.)
The definitive diagnostic test for OSA is polysomnography. The presence of sleep apnea is defined as 5 or more apneic events (cessation of airflow lasting 10 seconds or longer despite continued respiratory effort) per hour or 15 or more hypopneic events (decrease in airflow of more than 50% lasting 10 seconds or longer) per hour during a 7-hour sleep study.[108] The apneic-hypopneic index is the total number of apneic or hypopneic events (or both) per hour during sleep; the severity of OSA is directly related to the magnitude of this index.[96] Hypopneic and apneic events are associated with arousal from REM sleep and oxyhemoglobin desaturation. Daytime somnolence is a frequent complaint because sleep is fragmented such that the patient cannot sustain adequate intervals of REM sleep. Concomitant with apneic/hypopneic events, sympathetic nervous system activation occurs in response to hypoxemia.
Detection of OSA in obese patients presenting for bariatric surgery may be important for two reasons. First, patients with OSA are more sensitive to the consequences of the depressant effects of hypnotics and opioids on airway muscle tone and respiration.[110] Postoperative parenteral or neuraxial opioid administration to obese patients with OSA carries the potential for fatal or near-fatal respiratory misadventure.[111] [112] More intense postoperative nursing care and monitoring may be indicated when symptoms or signs of sleep apnea exist and either systemic or neuraxial opioids are necessary for postoperative pain control.[111] Second, OSA is associated with difficulty performing laryngoscopy[113] [114] and difficult mask ventilation.[115] In addition, obese patients have reduced oxygen stores because of their diminished expiratory reserve volume.[116] [117] The combination of these factors sets the stage for airway catastrophe. Because a large percentage of obese patients have sleep apnea, identification of those who probably do not have the condition may be more appropriate; in fact, some experts recommend presuming that all morbidly obese patients presenting for bariatric surgery have OSA. Thus, they recommend that all preoperative patients scheduled for bariatric surgery be trained in the use of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP).[118]
In the absence of previous polysomnographic testing, the presence and severity of OSA in a preoperative bariatric surgical patient are most conveniently assessed by the history. Useful information can often be elicited from the patient's roommate or sleeping partner. Historical information presumptive of sleep apnea includes habitual snoring, interrupted breathing during sleep (apneic spells followed by short gasps, grunts, or resuscitative snorting), impaired daytime performance, morning headache, and irritability.[96] Systemic hypertension[107] [109] and increased neck circumference (>40 to 42 cm at the cricoid cartilage)[108] [119] are consistent with the presumptive diagnosis of OSA. Other abnormalities associated with OSA that are detected by physical examination are somnolence and physical signs of difficult mask airway or difficult intubation (e.g., Mallampati class III or IV, hypognathia, decreased thyromental distance). [110] [112] [114]
Polysomnographic testing might be indicated in morbidly obese preoperative patients who habitually snore and report daytime somnolence or have observed periods of interrupted breathing during sleep (or both).[96] However, morbid obesity and symptoms of OSA per se are not indications for preoperative pulmonary function testing and room-air blood gas analysis. In the absence of uncharacterized symptoms or signs of pulmonary disease, these tests have neither predictive value nor utility in optimizing the postoperative management or outcome of bariatric surgery patients.[111] [120] [121] [122]
Obesity per se is not a common cause of chronic respiratory insufficiency. [123] Clinically significant impairment in gas exchange and pulmonary function are more common when chronic obstructive pulmonary disease (COPD) and obesity coexist. The two conditions together cause greater impairment in gas exchange than expected from simple summation of the impairment caused by each pathophysiologic process.[124] As in all patients, the presence of concomitant pulmonary system disease may be detected by a smoking history and symptoms or signs such as coughing, wheezing, or dyspnea on exertion.
In obese patients, chronic daytime hypoxemia is a better predictor of pulmonary hypertension and cor pulmonale than the presence and severity of OSA are.[125] [126] [127] Room-air pulse oximetry may be a useful, noninvasive means to screen patients for daytime hypoxemia, particularly if measured and compared in both the upright and supine positions. A supine room-air SpO2 of less than 96% may merit further investigation (e.g., pulmonary function tests, room-air ABGs, chest radiographs, and echocardiography). An elevated hematocrit may also be a clue to chronic hypoxemia.
A syndrome characterized by chronic daytime hypoventilation, dubbed the obesity-hypoventilation syndrome (OHS), can develop in a subgroup of obese patients. [123] OHS is also associated with chronic daytime hypoxemia (PO2 <65 mm Hg), conveniently detected during the preoperative visit by routine measurement of room-air pulse oximetry. Sustained hypercapnia (PCO2 >45 mm Hg) in an obese patient without significant obstructive pulmonary disease is diagnostic for this syndrome. These patients are typically extremely obese (BMI >40 kg/m2 ), and the likelihood of OHS increases strongly as BMI increases.[128] The precise underlying pathophysiology of OHS is unclear and probably multifactorial; however, chronic alveolar hypoventilation may be attributed to the compressive effect of extreme adiposity on the thoracic cage and diaphragm.[106] [123] Most obese patients with OHS also have OSA; nevertheless, obese patients with OSA do not commonly have OHS.[106] [123] Patients on the "severe" end of the OHS spectrum who have signs and symptoms of cor pulmonale are termed "pickwickian."[106] In one study of obese patients with OSA but without airflow obstruction, 56 of 58 patients with chronic daytime hypoxemia were proved by testing to have OHS.[127]
The reason to be wary of and to try to diagnose whether obesity coexists with either OHS or COPD is that such a combination often causes chronic daytime hypoxemia. Chronic daytime hypoxemia leads to pulmonary hypertension, right ventricular hypertrophy, or right ventricular failure (or any combination of these sequelae). These conditions (pickwickian) are associated with increased perioperative morbidity and mortality, and assessment of these patients may require extensive testing to guide preoperative medical optimization and postoperative management.[106] [109] [110] [129]
Prevalent ECG findings in obese patients are low QRS voltage, left ventricular hypertrophy or strain, left atrial abnormality, and T-wave flattening in the inferior and lateral leads.[130] ECG evidence in the obese of right ventricular hypertrophy or strain, right axis deviation, right bundle branch block, or P pulmonale is not common in the absence of pulmonary hypertension and cor pulmonale.
Morbid obesity with minimal or no coexisting pulmonary conditions (e.g., no OHS or COPD) will be referred to as "simple" obesity. In simple obesity, the pathophysiology of mild alterations in daytime gas exchange and pulmonary function may also result from compression and restriction of the chest wall and diaphragm by excess
Whereas vital capacity in simple obesity is reduced to 90% of control, it is reduced to 60% of normal in OHS.[134] The additional reduction in lung volume seen in OHS may be sufficient to cause marked increases in distal airway resistance. Airway resistance, increased by 133% in simple obesity, is increased by 650% in OHS.[134] In comparison to simple obesity, forced expiratory volume in 1 second (FEV1 ) and the maximum expiratory flow rate and maximum voluntary ventilation are reduced by 40% [134] in OHS. Accompanying these physiologic changes are the mechanical disadvantages of an overstretched diaphragm. In combination, these factors may lead to chronic respiratory muscle fatigue and the chronic hypoventilation characteristic of OHS.[106] In addition, the reduced lung volume associated with OHS may further impair ventilation-perfusion matching and cause decreases in PaO2 and increases in the A-a gradient greater than those of simple obesity. However, most of the reduction in PaO2 in OHS patients is postulated as being due to alveolar hypoxia from hypercapnia. [106]
Especially in patients with OHS, the supine position further reduces lung volume and increases distal airway resistance.[116] [131] Ferretti and associates[99] found that orthopnea in obese patients may be attributable to these physiologic causes. During the preoperative assessment, the extent of dyspnea that the patient experiences in the supine position may yield useful information.
In the perioperative setting, the reduction in chest wall and diaphragmatic muscle tone after induction of general anesthesia and skeletal muscle relaxation further impairs oxygenation. In simple obesity, the net effect may reduce ERV and FRC to less than 50% of preinduction values, thereby excluding even more alveoli from effective gas exchange.[131] In addition, reduction of ERV and FRC predisposes to atelectasis and limits effective clearance of secretions in the postoperative period.
ERV is the primary source of oxygen reserve during apnea. Therefore, in an obese patient, preoxygenation is less effective, and after apnea, the time to hemoglobin desaturation below 90% is reduced.[135] [136] In an obese, anesthetized, relaxed patient, the combination of a reduced "apneic oxygenation reserve" and the likelihood of difficultly with positive-pressure mask ventilation amplifies the potential for a hypoxemic misadventure. [137] [138] Elective awake tracheal intubation may be the safest approach if a patient scheduled for bariatric surgery has signs indicating a potential for difficult intubation, such as poor visualization of the posterior pharyngeal wall.[139] Positioning the patient with a roll under the scapulas and an occipital rest and asking the patient to fully extend at the atlanto-occipital joint before induction may facilitate awake or conventional laryngoscopy and intubation.
After major open abdominal surgery and without postoperative oxygen supplementation, even normal patients experience hypoxemia (SpO2 <90%) that persists several days and is most prevalent and severe on the night of the second postoperative day.[140] On the first postoperative day after open bariatric surgery, 75% of obese patients had a PaO2 less than 60 mm Hg; PaO2 averaged 14 mm Hg less than preoperative baseline measurements.[141] Although some improvement was seen, oxygenation remained significantly less than baseline during the subsequent postoperative days. These facts emphasize the importance of obtaining a preoperative assessment of baseline oxygenation (by pulse oximetry) and preparing for specific treatments should preoperative values minus 14 mm Hg be hazardous.
More extensive pulmonary function tests and preoperative treatment of any treatable abnormality (e.g., infectious and bronchospastic components of pulmonary disease) may be indicated for obese patients who smoke or have pulmonary symptoms (e.g., chronic cough, sputum production, wheezing, shortness of breath at rest or on minor exertion). Such evaluation may be indicated even for laparoscopic bariatric surgery. Although laparoscopic bariatric surgery may have fewer adverse effects on postoperative pulmonary gas exchange and require less postoperative analgesia, there is always the potential that the surgeon will need to convert to an "open" procedure. However, if an open procedure is likely and the obesity is severe, ABGs might also be analyzed to quantitate the degree of hypoventilation and aid in assessing the most appropriate time to extubate the trachea.
Postoperative continuous oxygen supplementation and maintenance of the semirecumbent or upright posture[142] might be a prudent strategy for all bariatric surgery patients. However, oxygen therapy alone may not be adequate, as determined by coexisting OSA, COPD, or OHS; postoperative opioid analgesic needs; and preexisting hypoxemia or orthopnea. If a need for opioid analgesics is anticipated in obese patients with OSA, OHS, COPD, the baseline SPO2 is less than 96%, or a history of orthopnea is elicited, postoperative CPAP or BiPAP therapy might be useful.
Nasal CPAP, a treatment of OSA since 1981, acts by "splinting" the airway during inspiration.[143] Given a patient compliance rate of 50% to 80%, it may be prudent to question whether the patient will accept this nocturnal therapy.[143] In normal-weight patients undergoing major abdominal surgery, nasal CPAP plus supplemental oxygen was not shown to decrease hypoxemic events the first postoperative night.[144] However, in surgical patients with OSA, postoperative nasal CPAP therapy has been shown to prevent episodic apneic events associated with fluctuations in BP.[145]
BiPAP, a more recent refinement of respiratory therapy, combines CPAP with additional inspiratory pressure support. Prophylactic BiPAP (12 cm H2 O inspiratory pressure, 4 cm H2 O expiratory pressure) during the first 24 to 48 hours after bariatric surgery significantly
It is unclear whether it is appropriate to delay bariatric surgery for aggressive optimization of airway status and oxygenation with CPAP or BiPAP therapy. Rennotte and colleagues[148] did not observe any major postoperative respiratory complications in 14 patients treated with nasal CPAP for up to 3 weeks before surgery, but without a prospective control group, it could not be determined whether complications would have occurred without such preparation. Two to three weeks may be necessary to not only maximize medical benefits but also allow sufficient time for patients unfamiliar with CPAP or BiPAP therapy to acclimate to nocturnal use of the device. Three weeks of nightly CPAP treatment before bariatric surgery improved the left ventricular ejection fraction and after-load in obese patients with coexisting heart failure.[149] Eight weeks of preoperative nasal CPAP therapy may be required to treat hypertension secondary to OSA.[150] After surgery, treatment should be applied as early as possible after extubation.[129]
Although assessment of venous status might seem strange as the first priority on cardiovascular evaluation, its importance and prominence become obvious when one looks at mortality data. Venous emboli that enter the pulmonary circulation are the major source of pulmonary dysfunction leading to the 1% to 2% 30-day mortality rate. Most mortality in the 30-day perioperative period after bariatric surgery is due to pulmonary embolism (this cause of mortality is three or more times more frequent than anastomotic leak with subsequent sepsis). Various drug regimens to decrease the thrombotic tendency have been attempted, but there is no agreement. The use of low-molecular-weight heparin might limit postoperative pain therapy options, and preoperative administration of aspirin and subsequent warfarin (Coumadin) to an international normalized ratio (INR) of 2.3 might be the current treatment of choice. The use of warfarin, a vitamin K antagonist, can be problematic postoperatively inasmuch as most patients after Roux-en-Y gastrojejunostomy malabsorb fat and fat-soluble substances, including vitamin K. This malabsorption makes the dose of warfarin difficult to control, and daily adjustment will often be required for at least a few weeks. Nevertheless, preoperative evaluation for venous stasis is difficult; many surgeons insist on preoperative exercise for at least an hour of walking or bicycle exercise 3 days a week for 6 weeks and the absence of leg symptoms (pain, soreness, or redness). Other surgeons or groups require 30 minutes of walking each day (see later for psychological factors that determine success). Factors that decrease the risk for venous stasis in multiple patients include preoperative exercise and antithrombotic drug and perhaps stocking prophylaxis, nonpolycythemic hematocrit, increased cardiac output, and early ambulation. Thus, evaluation of exercise status and drug therapy, absence of preexisting venous disease or signs or symptoms of venous disease, optimal hydration, and perioperative drug therapy all aimed at early ambulation might be the goals of this area of evaluation and prophylaxis. However, whatever is done, it should be remembered that this area is the major determinant of perioperative survival.
Cardiac output must increase approximately 0.01 L/min to perfuse each kilogram of adipose tissue. As a result, obese patients often have hypertension, which can cause cardiomegaly and left ventricular failure. Care should be taken to use a BP cuff of correct size when quantitating the degree of hypertension present.
The obese may have limited cardiac reserve and poor tolerance for stress induced by hypotension, hypertension, tachycardia, or fluid overload associated with the preoperative and preprocedure period. Massively obese patients with carbon dioxide retention are called pickwickian, alveolar hypoventilation being the hallmark of this condition. Other components of the pickwickian syndrome are somnolence, hypoxemia, failure of the right side of the heart, and secondary polycythemia. Many of these patients have right ventricular failure (also see Chapter 32 for monitoring considerations). Thus, preoperative and preprocedure assessment should include not only history taking and physical examination with an emphasis on drug therapy and cardiopulmonary problems, but also an ECG examination looking specifically for left or right ventricular hypertrophy, ischemia, and conduction defects.
Obese persons may metabolize lipophilic drugs to a greater degree (and for longer periods) than their thin counterparts. More fluorine is produced from enflurane given to obese patients than to thin ones. One would assume that responses to drugs stored in fat (e.g., narcotics, barbiturates, volatile anesthetics) would be prolonged in the obese. There is no evidence, however, that use of the more soluble anesthetics delays recovery time in obese subjects. The dose requirements of pharmacologic drugs used for analgesia and airway management are also altered by significant overweight status (BMI >27.5). Increased body fat increases the volume of distribution of sufentanil and slows its elimination. In one study, the elimination half-life of sufentanil was 208 minutes for eight obese patients (mean weight, 94 kg) versus 135 minutes for eight controls (mean weight, 70 kg).[151] Similarly, muscle relaxants that depend on hepatic blood flow for elimination (i.e., pancuronium, vecuronium, and rocuronium) appear to have dosage requirements that are directly proportional to body surface area, as well as longer twitch recovery times. The 25% to 75% twitch recovery time for vecuronium was 38.4 minutes for obese patients (mean weight, 93 kg) versus 17.6 minutes for nonobese patients (mean weight, 61 kg).[152]
The anesthesiologist should also be aware of conditions caused by remedies other than bariatric surgery used to treat obesity.[153] Drastic dieting can produce acidosis, hypokalemia, and hyperuricemia; protein hydrolysate
Drug treatment of obesity also has implications for the anesthesiologist. Amphetamines (and probably mazindol) given acutely increase anesthetic requirements; by contrast, amphetamines administered chronically decrease anesthetic requirements (see the section on chronic drug therapy). Amphetamines may interfere with the action of vasoactive drugs given to treat hypotension or hypertension.
Because many obese patients have tried drug therapies, the anesthesiologist should consider asking questions about the use of adjuvants. If such drugs have been used, the anesthesiologist might consider auscultation and echocardiography to search for mitral valve regurgitant lesions because some dietary aids of the past (notably "phen-fen") have been associated with these conditions. Other dietary herbs are also known to cause liver dysfunction, so searching for the use of these adjuvants may be important. Fenfluramine (a drug that inhibits the serotonergic system) by itself may decrease both anesthetic requirements and BP.
Psychological evaluation is crucial for patient success after bariatric surgery. The year after surgery is not easy, let alone the week after. The patient must be sapient and engaged and must be able to have the discipline to keep a food diary. The evaluation of the anesthesiologist can provide valuable clues—has the patient been walking daily, for example. The patient needs to be emotionally stable. Choices that the anesthesiologist can uncover that correlate with failure are street drug abuse, unaddressed major psychiatric disorders/illness, compulsive eating disorder, and the diagnosis of fibromyalgia or chronic fatigue syndrome. If such are searched for and found on preoperative evaluation, communication among all, including the surgeon, can spare the health care team much frustration and save the patient much distress.
Other features of obesity are of prognostic and perioperative importance to the anesthesiologist as well. Because of excessive and extensive subcutaneous fat and large size of the extremities, proper positioning of the patient, placement of monitoring devices, and establishment of intravenous sites are more difficult to accomplish, and BP (and the choice of cuff size) is less easy to ascertain than for patients of normal weight.
Preoperative consideration of the positioning of obese patients may eliminate some postoperative problems. In a retrospective study, Warner and colleagues[155] found that patients whose BMI was greater than 38 kg/m2 had a 29% incidence of postoperative ulnar neuropathy versus a 1% incidence in controls. Upper brachial plexus root injury may result from extreme rotation of the head and cervical spine to the opposite side.[156] Lower root injury may result from hyperabduction of the arm on the same side as the injury. Preoperatively, one may assess sites vulnerable to pressure injury. Intraoperatively, pressure point checks and repositioning periodically make good sense.
Preoperative evaluation of the deep venous system of the legs is difficult in the obese. (Even more sponges and instruments are left in the obese.) Routine prophylaxis for deep venous thrombosis is commonly initiated preoperatively, a prudent practice given that obesity is a major risk factor for sudden postoperative death from massive pulmonary thromboembolism. Anticoagulant prophylaxis is likely to be ordered by the surgeon, and such therapy must be considered if a central neuraxial catheter is to be inserted and used for postoperative analgesia. Furthermore, the incidence of wound infection, deep vein thrombosis, and pulmonary embolism is increased; the latter two should probably be guarded against with subcutaneous heparin and early ambulation. Thus, a knowledge of or discussion with the specific surgeon of preferences for postoperative prophylaxis of deep vein thrombosis and its consequences and preparation of the patient for this plan may be an important aspect of the preoperative meeting of the anesthesiologist and patient.
Many endocrine and metabolic abnormalities occur in patients with anorexia nervosa, a condition characterized by starvation to the point of 40% loss of normal weight, hyperactivity, and a psychiatrically distorted body image. Many anorectic patients exhibit impulsive behavior, including suicide attempts, and intravenous drug use is much more common than in the general population. Acidosis, hypokalemia, hypocalcemia, hypomagnesemia, hypothermia, diabetes insipidus, and severe endocrine abnormalities mimicking panhypopituitarism need attention before anesthesia and surgery. Similar problems occur in bulimia (bulimorexia), a condition that may affect as many as 50% of female college students[157] and is even unintentionally present in many elderly.[158] As in severe protein deficiency (kwashiorkor), anorexia nervosa and bulimia may be accompanied by ECG alterations, including a prolonged QT interval, atrioventricular (AV) block, and other arrhythmias; sensitivity to epinephrine; and cardiomyopathy. [157] [159] Total depletion of body potassium makes the addition of potassium to glucose solutions useful; however, fluid administration can precipitate pulmonary edema in these patients. Esophagitis, pancreatitis, and aspiration pneumonia are more frequent in these patients, as is delayed gastric emptying. Thus, invasive monitoring (radial artery and pulmonary artery catheterization) may be indicated for anorectic, bulimic, and malnurtured patients requiring emergency surgery. Elective surgery should probably be delayed until the abnormalities are treated.
|
|
|
|
|
|
|
|
|
|
|
|
|