|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This section reviews the interventions, both electrical and pharmacologic, used in treatment of the various forms of cardiorespiratory arrest. Included is a discussion of the major beneficial and adverse actions of the drugs used in ACLS. The clinical applications of some of these drugs have already been presented in the review of recognition and management of "prearrest" or potentially life-threatening events.
For therapeutic purposes, it is reasonable to consider pulseless VT and VF as the same entity in need of the same interventions. It is fortunate that this is the most common form of cardiac arrest because it is the most treatable and it is the arrhythmia that yields the greatest likelihood of immediate and long-term survival, both in the hospital and out of the hospital.[158] This finding has accelerated the extension of early intervention in VF to levels of out-of-hospital emergency care lower than emergency medical technicians, even to first responders with minimal training.[159] The likelihood of significant salvage by early defibrillation also accounts for the growing interest in and distribution of AEDs[8] and the rapid increase in implantation of cardioverter-defibrillators[160] [161] [162] [163] [164] [165] in patients at risk of cardiac arrest from VF. It is obvious that physicians involved in caring for patients in the hospital are assumed to know how to intervene in the event of cardiac arrest, how to operate defibrillators, and how to administer drugs rationally, especially in the presence of VT/VF, when survival is likely with rapid and correct treatment.
Despite rare reports of apparently spontaneous termination of VF, the required and definitive intervention is rapid defibrillation. Two practical and important considerations enter into every attempt to defibrillate patients in VF. The first is the energy output of the defibrillator.[166] Termination of VF is critically dependent on the amount of energy available from a defibrillator, and it is therefore necessary that output be checked at regular intervals. Guidelines for proper maintenance of defibrillators are available.[167]
The second practical consideration is resistance to current flow during shock delivery. Current flow is inversely related to resistance; excessive resistance because of poor technique can impede the transthoracic flow of current adequate to induce defibrillation.[166] The major operator-controllable variables that can reduce impedance during defibrillation are proper electrode position, firm pressure against each hand-held paddle (11 kg per paddle), and an optimal electrode-chest wall coupling medium such as electrode paste or the use of self-adhesive electrode pads.[166] Preapplied electrode pads are particularly advantageous in the operating room when patient position precludes rapid access to the chest for standard paddle placement. Finally, defibrillation should occur at the end of expiration during rescue breathing to minimize impedance.
In the operating room or ICU, when cardiac arrest is witnessed and a monitor reveals the mechanism to be pulseless VT or VF, biphasic defibrillation using up to three nonescalating shocks of 150 J should be administered if the arrhythmia persists. In circumstances in which a monophasic defibrillator is available, a 200-J shock should be delivered immediately, followed by a second shock of 200 to 300 J and, if necessary, a third shock of 360 J. In biphasic or monophasic defibrillation, the series of three shocks should be delivered in rapid succession as needed. If a cardiac arrest is witnessed but not monitored and a defibrillator is not available, a single precordial thump can be applied before beginning CPR and while awaiting arrival of the monitor-defibrillator. If at any time VF recurs after successful conversion, the series of up to three shocks should be repeated. This sequence of rapidly repeated defibrillatory shocks for persistent VF underscores the primacy of this treatment of VF over all other interventions. If VT persists despite this initial treatment, pharmacologic therapy becomes necessary. It is assumed that the anesthesiologist has already controlled the airway and ventilation by endotracheal intubation and ventilation with 100% oxygen. In situations where endotracheal intubation is not in place at the time of cardiac arrest, defibrillation attempts take precedence over endotracheal intubation if the defibrillator is immediately available.
Although nonescalating biphasic waveform technology is the standard for AED, the optimal biphasic, non-automated defibrillator waveforms and first-shock energies for termination of VF are not known. Current experience
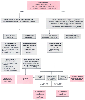 Figure 78-13
The tachycardia overview algorithm. bpm, beats per minute;
CHF, congestive heart failure; ECG, electrocardiogram; SVT, supraventricular tachycardia;
VT, ventricular tachycardia; WPW, Wolff-Parkinson-White syndrome. (Redrawn
with modification from American Heart Association in collaboration with International
Liaison Committee on Resuscitation: Guidelines 2000 for Cardiopulmonary Resuscitation
and Emergency Cardiovascular Care: International Consensus on Science, Parts 1–12.
Circulation 102(Suppl I):I–1, 2000.)
Figure 78-13
The tachycardia overview algorithm. bpm, beats per minute;
CHF, congestive heart failure; ECG, electrocardiogram; SVT, supraventricular tachycardia;
VT, ventricular tachycardia; WPW, Wolff-Parkinson-White syndrome. (Redrawn
with modification from American Heart Association in collaboration with International
Liaison Committee on Resuscitation: Guidelines 2000 for Cardiopulmonary Resuscitation
and Emergency Cardiovascular Care: International Consensus on Science, Parts 1–12.
Circulation 102(Suppl I):I–1, 2000.)
 Figure 78-14
Wide-QRS complex tachycardia at a rate of 160/min. A,
Precordial lead 5. B, Lead 2. Atrioventricular dissociation
is present, evident by the P wave preceding the third QRS complex, thus confirming
this abnormality to be ventricular tachycardia. (From Wagner SR IV, Johnson
SS, White RD: Intraoperative ventricular tachycardia responsive to adenosine. Anesth
Analg 82:1086, 1996.)
Figure 78-14
Wide-QRS complex tachycardia at a rate of 160/min. A,
Precordial lead 5. B, Lead 2. Atrioventricular dissociation
is present, evident by the P wave preceding the third QRS complex, thus confirming
this abnormality to be ventricular tachycardia. (From Wagner SR IV, Johnson
SS, White RD: Intraoperative ventricular tachycardia responsive to adenosine. Anesth
Analg 82:1086, 1996.)
If a return of spontaneous circulation has not occurred by the third defibrillation attempt, pharmacologic interventions should accompany resuscitation efforts. For VF and pulseless VT, epinephrine and vasopressin are indicated. Assuming that a peripheral intravenous line is present, initial drug injections can be given by this route, but if cardiac arrest persists, a central venous catheter should be inserted to ensure more rapid drug delivery and attainment of higher peak arterial concentrations more quickly than with peripheral injection.[100] Because of evidence that glucose administration during global cerebral ischemia can worsen neurologic outcome in survivors,[169] [170] [171] [172] lactated Ringer's solution or normal saline should be used instead of glucose-containing solutions.
Previous clinical impressions regarding the beneficial effects of epinephrine during cardiac arrest, regardless of the underlying rhythm, have been substantiated.[173] [174] [175] [176] [177] The
 Figure 78-15
Adverse response to verapamil. Tachycardia thought to
be atrial flutter was present initially (A). After
the injection of 5 mg verapamil, the rate accelerated and the patient became unresponsive,
necessitating cardioversion (B). The first rhythm
was ventricular tachycardia. Cardiovascular collapse is a potential complication
of verapamil injection when the tachycardia is ventricular in origin.
Figure 78-15
Adverse response to verapamil. Tachycardia thought to
be atrial flutter was present initially (A). After
the injection of 5 mg verapamil, the rate accelerated and the patient became unresponsive,
necessitating cardioversion (B). The first rhythm
was ventricular tachycardia. Cardiovascular collapse is a potential complication
of verapamil injection when the tachycardia is ventricular in origin.
Because of the critical role of potent vasoconstriction in the beneficial action of epinephrine, phenylephrine has been studied as well.[174] [182] [183] Comparison of phenylephrine and epinephrine in experimental cardiac arrest has shown no evident benefit gained from using phenylephrine in terms of changes in blood flow,[173] [183] somatosensory evoked potentials,[173] or neurologic or cardiovascular outcome.[175] Thus, epinephrine remains the drug of choice at this time.
Based on experimental observations of apparent hemodynamic benefit from large doses of epinephrine (up to 0.2 mg/kg),[184] [185] numerous clinical reports and studies have assessed the impact of high-dose epinephrine on such end points as restoration of spontaneous circulation and discharge from the hospital.[186] [187] [188] [189] [190] [191] [192] Although some investigators have observed an increased frequency of restoration of spontaneous circulation after
Vasopressin was added to the treatment algorithm of VF and pulseless VT based on experimental and clinical data suggesting that it has beneficial effects on vital organ perfusion during cardiac arrest.[193] [194] Endogenous plasma vasopressin levels were noted to be significantly higher in patients successfully resuscitated from out-of-hospital cardiac arrest than in those who died, thus suggesting that the human body discharges this hormone under circumstances of physiologic stress.[195] [196] Vasopressin acts by direct stimulation of smooth muscle V1 receptors, and such stimulation results in prolonged smooth muscle constriction even in the presence of severe acidosis, which might maintain coronary perfusion pressure and contribute to improved success with resuscitation. [197] [198] In one study, vasopressin administered to patients with refractory cardiac arrest led to increased blood pressure and, in some, return of spontaneous circulation.[199] However promising these results are in suggesting a potential benefit for vasopressin in cardiac arrest, no study has demonstrated significant benefit resulting in hospital discharge. In Guidelines 2000, the use of vasopressin is endorsed as an alternative vasopressor to epinephrine during refractory VF.[200] It may have applications in other circumstances of cardiac arrest (e.g., pulseless electrical activity [PEA]) and vasodilatory shock; however, evidence is currently insufficient to endorse its use. Because of its long half-life (10 to 20 minutes) with respect to epinephrine (3 to 5 minutes), vasopressin is administered in a one-time dose of 40 U intravenously or interosseously.
If VF persists after three initial defibrillations and injection of epinephrine, vasopressin, or both, a fourth defibrillation using 360 J (monophasic) or maximal biphasic energy should be attempted. If VF persists after these interventions, medications with antifibrillatory effects should be chosen. Lidocaine (1.5 mg/kg intravenously) and amiodarone (300 mg intravenously) are acceptable choices in this situation. Lidocaine has been shown to enhance intraoperative ventricular defibrillation in cardiac surgery by permitting defibrillation with fewer shocks of lower energy and current.[201] This concept is controversial, however, because other investigators have observed an elevation in the defibrillation threshold with blood concentrations of lidocaine that may occur during cardiac arrest and resuscitation.[202] [203] [204] The international Guidelines 2000 recognizes that lidocaine has not been subjected to randomized
 Figure 78-16
A and B,
Hyperkalemic ventricular tachycardia with a sine wave morphologic pattern. The serum
potassium concentration was 7.8 mEq/L.
Figure 78-16
A and B,
Hyperkalemic ventricular tachycardia with a sine wave morphologic pattern. The serum
potassium concentration was 7.8 mEq/L.
Amiodarone produces prolongation of action potentials and refractoriness in all cardiac tissue. These effects may contribute to its potential use in refractory VF. In the Amiodarone in Out-of-Hospital Resuscitation of Refractory Sustained Ventricular Tachycardia (ARREST) study, Kudenchuk and colleagues[206] reported the results of a randomized, prospective study in which amiodarone, 300 mg intravenously, or placebo was administered to victims of out-of-hospital cardiac arrest with persistent VF after three attempts at defibrillation and 1 mg epinephrine intravenously. The study demonstrated significant increases in survival to hospital admission in the amiodarone-treated group but was not able to demonstrate any difference in hospital discharge rates. In the Amiodarone versus Lidocaine in Pre-hospital Ventricular Fibrillation Evaluation (ALIVE) study, Dorian and colleagues[207] reported the results of a double-blind, controlled clinical trial comparing amiodarone with lidocaine in patients experiencing out-of-hospital cardiac arrest in Toronto, Canada. Their results demonstrated that amiodarone was superior to lidocaine in terminating persistent VF in the out-of-hospital setting. Like the ARREST study, the ALIVE study was able to demonstrate the improvement induced by amiodarone only in survival to hospital admission, not to discharge, when compared with control or lidocaine therapy. Based on these studies, greater consideration for the use of amiodarone rather than lidocaine seems prudent in the management of patients experiencing persistent VF.
In cardiac arrest that does not respond to the initial measures (intubation, ventilation with 100% oxygen, defibrillation, epinephrine, and antiarrhythmic drugs), sodium bicarbonate can be considered if specific conditions are present such as preexisting metabolic acidosis, severe documented metabolic acidosis assessed during resuscitation attempts, and hyperkalemia. If hyperkalemia is known or suspected to be present, sodium bicarbonate should be administered promptly ( Fig. 78-16 ). Despite its long history of early and frequent administration in cardiac arrest, there is still no convincing evidence that it confers any benefit in survival from VF.[208] [209] By contrast, its adverse effects are well documented, including severe plasma hyperosmolality, [210] [211] paradoxical cerebrospinal fluid acidosis,[212] and CO2 generation.[210] [213] It is this last effect that has been primarily responsible for its infrequent role in cardiac arrest treatment algorithms.
During cardiac arrest with severely reduced pulmonary blood flow and therefore CO2 transport and elimination, CO2 is retained in mixed-venous blood and tissues, and a disparity between pH and PCO2 in arterial and mixed-venous blood is thus produced.[213] Sodium bicarbonate is a rapid and potent CO2 generator, which can be observed in arterial blood, as well as expired air, after intravenous injection. Sodium bicarbonate would only add to the CO2 load and could worsen mixed-venous (and presumably tissue) acidosis. Myocardial hypercapnic acidosis has been confirmed to exist during experimental cardiac arrest and resuscitation,[214] and it is not reversed with sodium bicarbonate or other buffer agents.[215] At least with regard to the heart, increases in tissue PCO2 produce progressive decreases in the performance of ischemic myocardium. Clinically, one may infer that intractable PEA could be a consequence, even if VF were eliminated. Myocardial hypercapnic acidosis decreases the success of restoration of spontaneous circulatory function and survival.[216]
In light of all these observations, sodium bicarbonate should not be used routinely in the treatment of cardiac arrest. It can be considered after the foregoing electrical and pharmacologic measures have been instituted, if preexisting metabolic acidosis is present, or if severe documented metabolic acidosis develops during the arrest.[217] A review of acid-base derangements during cardiac arrest[218] [219] suggests that sodium bicarbonate may be beneficial in restoring spontaneous circulation after prolonged (15 minutes) VF cardiac arrest based on experimental observations. Clinically, if this drug is used, an initial dose of 1 mEq/kg can be given, followed at 10-minute intervals by 0.5 mEq/kg. Of course, if a base deficit is documented on blood gas analysis, the amount of drug can be based on that measurement. Other antacids such as sodium carbonate,[217] carbicarb, [217] [220] and tribonat [217] [221] have been studied, but none is superior to sodium bicarbonate in clinical cardiac arrest. Monitoring both arterial and mixed-venous blood gases and pH during cardiac arrest helps define the derangements in acid-base equilibrium occurring in arrested humans and thereby leads to more rational antacid therapy.
PEA refers to a heterogeneous group of cardiac rhythm disorders, all characterized by pulselessness in the presence of some type of electrical activity other than VT or VF. Thus, included in this designation are traditional electromechanical dissociation, in which pulseless but organized electrical activity is present; idioventricular rhythms; ventricular escape rhythms; postdefibrillation idioventricular rhythms; and bradyasystole.
High priority must be given to identification of a possibly correctable cause of any form of PEA. For example, in a traumatized patient, hypovolemia, cardiac tamponade and tension pneumothorax with hypoxemia are possible causes. Intraoperatively or postoperatively, acute massive pulmonary thromboembolism should be considered. Idioventricular rhythms may accompany such derangements as severe hyperkalemia, acidosis, hypothermia, or overdose with drugs such as digitalis, β-blockers, calcium channel blockers, and tricyclic antidepressants.
In any of these forms of PEA, the most important point is that although initial temporizing measures such as injection of epinephrine or pacing may be needed to "tide the patient over," an immediate diagnostic assessment and redirection of treatment in a disorder-specific direction may permit salvage of any patient with PEA. Figure 78-17 provides the PEA treatment algorithm with potentially reversible causes contributing to the condition.
Complete and sustained absence of electrical activity is most often an irreversible and therefore terminal event caused by such derangements as uncorrected persistent hypoxia, severe hyperkalemia, massive drug overdose, myocardial infarction, or hypothermia. In most of these conditions, the asystole is irreversible and terminal, but at least a brief trial of interventions may be warranted in some patients. In specific instances such as hyperkalemia, known metabolic acidosis, or tricyclic antidepressant overdose, sodium bicarbonate should be administered early in the effort. Pacing is unlikely to be successful in restoring circulation.
Discharge survival rates after in-hospital cardiac arrest and resuscitation range from 8% to 21%, with most reports demonstrating an average survival rate of approximately 14%.[222] [223] [224] [225] [226] [227] [228] [229] [230] [231] [232] These reports usually include cardiac arrests in both ICUs and general wards. In a retrospective review of 668 cardiac arrests over a 3-year period, the discharge survival rate was 3.3% in ICU patients and 14.0% in non-ICU patients.[230] In a small retrospective study of 24 consecutive patients who had an intraoperative cardiac arrest between 1986 and 1994, the survival rate was 38%.[233] A primary cardiac event was presumed to be causative in 50%. An accompanying invited commentary pointed out that much of the credit for these favorable outcomes is attributable to advances in intraoperative management by anesthesiologists. In any case, it is certain that anesthesiologists well trained in resuscitation can play a decisive role in the management of patients with intraoperative cardiac arrest.
The most favorable outcome, as in out-of-hospital cardiac arrest, is observed with VF or VT.[222] [224] [225] [227] [228] [229] In a study comparing CPR techniques in 143 hospitalized patients experiencing asystolic or electromechanical dissociative arrest, there were no survivors to hospital discharge with intact neurologic function.[51] The duration of resuscitative attempts is a determinant of survival[62] [264] [265] [266] [267] [268] ; in one study, none of 179 patients in whom resuscitative efforts lasted longer than 30 minutes survived to be discharged.[227] Other variables that limit survival from in-hospital cardiac arrest include unwitnessed arrest,[230] sepsis,[230] cancer,[227] [230] renal failure,[227] and prearrest hypotension.[222] [227] Age is a major determinant of outcome.[230] In one study, no patients 70 years or older who experienced cardiac arrest and resuscitative attempts survived to discharge.[230] These data were obtained in a hospitalized population of relatively sick, aged male patients, and it is not possible to extrapolate these observations to a different hospital population. Nevertheless, these observations have provoked open and
 Figure 78-17
Pulseless electrical activity algorithm. ACS, acute
coronary syndrome; EMD, electromechanical dissociation; CPR, cardiopulmonary resuscitation;
IV, intravenous; OD, overdose; VF, ventricular fibrillation; VT, ventricular tachycardia.
(Redrawn with modification from American Heart Association in collaboration
with International Liaison Committee on Resuscitation: Guidelines 2000 for Cardiopulmonary
Resuscitation and Emergency Cardiovascular Care: International Consensus on Science,
Parts 1–12. Circulation 102(Suppl I):I–1, 2000.)
Figure 78-17
Pulseless electrical activity algorithm. ACS, acute
coronary syndrome; EMD, electromechanical dissociation; CPR, cardiopulmonary resuscitation;
IV, intravenous; OD, overdose; VF, ventricular fibrillation; VT, ventricular tachycardia.
(Redrawn with modification from American Heart Association in collaboration
with International Liaison Committee on Resuscitation: Guidelines 2000 for Cardiopulmonary
Resuscitation and Emergency Cardiovascular Care: International Consensus on Science,
Parts 1–12. Circulation 102(Suppl I):I–1, 2000.)
Resuscitation in the ICU is typically prompt and effective. Long delays in recognizing and treating cardiac arrest in general care areas of hospitals and outpatient medical facilities are common.[237] In an attempt to improve in-hospital resuscitation success rates in medical facilities (e.g., hospitals, clinics, outpatient diagnostic centers), Guidelines 2000 recommends the distribution of AEDs to ensure that less than 3 minutes pass between collapse and the first AED shock in most patients in medical facilities.[238] Rather than identifying and training central resuscitation teams, general care nurses and other hospital employees should be trained in use of the AED and authorized to initiate therapy when indicated. The Joint Commission on Accreditation of Healthcare Organizations (JCAHO), a U.S. regulatory agency working to improve patient care and safety, has included resuscitation response measures and procedures as an important area for review to ensure that victims of inhospital cardiac arrest are provided prompt interventions by well-trained providers with appropriate systems for response, data collection, review, and tracking over several years.[237]
The increasing application of do-not-resuscitate (DNR) orders in hospitalized patients has necessitated the development of specific guidelines for the management of such patients. The CPR and emergency cardiac care guidelines [6] addressed this issue a in section titled "Ethical Considerations in Resuscitation," and the American Medical Association Council on Ethical and Judicial Affairs developed guidelines for the use of DNR orders.[239] These documents provide helpful assistance in the use of DNR orders in hospitalized patients and should be consulted.
In an analysis of DNR orders in ICUs in which practice in the period 1988 to 1990 was compared with that in 1979 to 1982, it was observed that DNR orders were written earlier and more frequently in the more recent period.[240] These practice changes preceded implementation of the Patient Self-Determination Act, a finding suggesting acceptance of the limitations of treatment by both physicians and families that has resulted in more frequent and earlier application of DNR policies.
A unique aspect of applying DNR orders is posed by a patient with such orders who comes to the operating room, for example, for a palliative procedure. Should DNR orders be suspended for this period, or should they be modified in some way? These are difficult questions
| Maneuver | Infant (<1 yr) | Child (1–8 yr) |
|---|---|---|
| Airway | Head tilt-chin lift (unless trauma present) | Head tilt-chin lift (unless trauma present) |
|
|
Jaw thrust | Jaw thrust |
| Breathing |
|
|
| Initial | 2 breaths at 1–1 ½ sec/breath | 2 breaths at 1–1 ½ sec/breath |
| Subsequent | 20 breaths/min | 20 breaths/min |
| Circulation |
|
|
| Pulse check | Brachial/femoral | Carotid |
| Compression area | Lower half of sternum | Lower half of sternum |
| Compression width | 2 or 3 fingers | Heel of 1 hand |
| Depth | Approximately ⅓ to ½ the depth of the chest | Approximately ⅓ to ½ the depth of the chest |
| Rate | At least 100/min | 100/min |
| Compression | 5:1 (pause for ventilation) | 5:1 (pause for ventilation) |
| Airway obstruction by foreign body | Back blows/chest thrusts | Heimlich maneuver |
| (From American Heart Association in collaboration with International Liaison Committee on Resuscitation: Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: International Consensus on Science, Parts 1–12. Circulation 102(Suppl I):I–1, 2000.) | ||
|
|
|
|
|
|
|
|
|
|
|
|
|