|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Although it is commonly understood and accepted that all physicians, regardless of specialty, should be able to perform CPR, it must be emphasized that CPR almost invariably necessitates rapid interventional follow-up care with ACLS procedures. It is essential that anesthesiologists be capable of rendering such definitive follow-up intervention, whether in the operating room, ICU, emergency department, delivery room, or hospital ward. In the operating room, cardiac arrest is rare. In two recent studies, cardiac arrests occurring within 12 hours[93] and 24 hours[94] of the induction of anesthesia were reviewed for evaluating the role that anesthesia may have contributed to the cardiac event. The somewhat reassuring observation that intraoperative cardiac arrests are rare (1.1/10,000 and 1.4/10,000, respectively) does not dismiss the need for anesthesiologists to be thoroughly acquainted with ACLS equipment and interventions because when these methods are needed, they must be executed skillfully and decisively. The need for skillful and knowledgeable ACLS intervention in intraoperative cardiac arrest is recognized.[95] Failure to intervene rapidly with ACLS pharmacologic therapy was identified as a major cause of poor outcome in other reports of intraoperative cardiac arrest.[95] [96] It is evident that ACLS is a body of knowledge and skill with which anesthesiologists must be thoroughly familiar.
In a study using a computer program that simulates critical patient incidents such as cardiac arrest, it was observed that only 30% of participants, who consisted of anesthesiology residents, faculty, and private practitioners, managed a simulated cardiac arrest in accordance with AHA ACLS guidelines.[97] Time since the last ACLS training was noted to be an important predictor of proper management of simulated cardiac arrest. Of those trained in ACLS within 6 months preceding the assessment, 71% managed simulated cardiac arrests successfully, whereas successful management decreased to about 30% in those whose ACLS training occurred 6 months to 2 years earlier. No participant who had been trained in ACLS longer than 2 years before the assessment used ACLS guidelines correctly in the simulated cardiac arrest management.[97] This experience supports the position that some form of training and periodic retraining in ACLS are necessary to enable anesthesiologists as well as other physicians to maintain the level of knowledge and skill essential for management of cardiorespiratory arrest in accord with contemporary principles as incorporated in the ACLS training program. Because advanced airway control and ventilation have already been discussed, this section presents the remaining components of ACLS as they directly pertain to anesthesiology practice.
Prompt recognition and treatment of potentially life-threatening (prearrest) cardiac arrhythmias are essential components of ACLS (also see Chapter 34 ).[98] Early recognition and immediate pharmacologic intervention can frequently prevent the onset of fatal arrhythmias necessitating the full application of BLS and ACLS. In this context, supraventricular arrhythmias that may cause hemodynamic compromise are discussed first, followed by recognition and management of those that are ventricular in origin.
Supraventricular bradyarrhythmias may be sinus or junctional in origin, or they may be caused by second-degree (types I and II) or third-degree atrioventricular (AV) block. Sinus (or junctional) bradycardia and type I (AV nodal) second-degree block are usually manifestations of increased vagal tone. Sinus bradycardia and type I second-degree AV block (Wenckebach phenomenon) may be observed during high-dose narcotic anesthesia. During spinal anesthesia, reduced venous return and unopposed vagal tone may produce bradycardia and hypotension of sufficient severity to progress to cardiac arrest.[99] Treatment is indicated whenever the bradycardia, regardless of type, leads to a significant decrease in systemic arterial pressure, produces clinical signs of reduced cardiac output (or a decrease in measured output), or is accompanied by ventricular ectopic depolarization. Any of these signs should be taken as evidence of hemodynamic or electrophysiologic deterioration with the propensity to progress to lethal arrhythmias, either asystole or VF. Initial treatment is with atropine, 0.5 to 1.0 mg intravenously and repeated as needed at 3- to 5-minute intervals to 0.04 mg/kg.[100] If such treatment is ineffective in producing an increase in heart rate and hemodynamic (increased systemic pressure, cardiac output, or both) or electrophysiologic (elimination of ventricular ectopy) improvement, alternatives include external or transvenous pacing or, during spinal anesthesia, low-dose (0.2 mg) intravenous epinephrine.[101] The availability of intraoperative cardiac pacing equipment and techniques has diminished the need for isoproterenol for the treatment
Pulmonary artery catheters that permit the insertion of atrial or ventricular pacing probes are available. If such a catheter is in place, emergency pacing can be instituted rapidly. Pacing probes can be advanced into position quickly to permit rapid control of the cardiac rate by ventricular, atrial, or AV sequential pacing. Atrial pacing wires require an intact AV conduction system. Ventricular pacing is indicated when AV nodal conduction is disrupted.
External transcutaneous pacing can be used to rapidly treat atropine-resistant bradyarrhythmias. These devices have been described as safe and effective, and intraoperative experience has been favorable.[102] [103] Their greatest therapeutic benefit is likely to be for atropine-resistant bradyarrhythmias, and these devices should be available for emergency use along with a defibrillator. External pacing has not yet been shown to be useful in bradyasystolic cardiac arrest.
Transesophageal atrial pacing has been effective in treating intraoperative supraventricular bradyarrhythmias such as sinus or junctional bradycardia.[104] [105] In its present configuration, however, it cannot be used to pace the ventricles, and therefore it is not useful in the management of any form of bradycardia caused by AV conduction disturbances.
Supraventricular tachyarrhythmias include atrial flutter, atrial fibrillation, AV junctional tachycardia, multifocal atrial tachycardia, paroxysmal reentrant tachycardia, and other much less frequent arrhythmias. Paroxysmal supraventricular tachycardia (PSVT), atrial fibrillation (or flutter) with rapid ventricular rates, and multifocal atrial tachycardia are discussed here because not only can they produce hemodynamic compromise, they can also present diagnostic and therapeutic challenges.
An example of PSVT is shown in Figure 78-3 . If such a tachyarrhythmia produces hemodynamic deterioration, cardioversion is the treatment of choice, beginning at 50 J and progressing to 100, 200, 300, and 360 J if needed. If the patient is hemodynamically stable, vagal maneuvers can be attempted (e.g., Valsalva maneuver in awake patients) before initiating pharmacologic interventions. If
 Figure 78-3
Paroxysmal supraventricular tachycardia. The rate is
250 beats/min with no clearly identifiable P waves.
Figure 78-3
Paroxysmal supraventricular tachycardia. The rate is
250 beats/min with no clearly identifiable P waves.
Adenosine's very short half-life (<5 seconds) is both advantageous and disadvantageous: side effects such as flushing, dyspnea, and chest pain are short lived, but the tachyarrhythmia may recur and necessitate the use of another drug. The AV nodal-blocking action of adenosine is antagonized by theophylline or related methylxanthines and is potentiated by dipyridamole and carbamazepine ( Fig. 78-6 ).[106] [107] [108] [121] [122] A suggested scheme for the use of adenosine, including dosing adjustments, is presented in Table 78-2 .
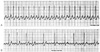 Figure 78-4
Supraventricular tachycardia at a rate of 180 beats/min
is present; the origin of the tachycardia is not evident (A).
After injection of 6 mg adenosine, atrial flutter waves are clearly visible, thereby
establishing the diagnosis (B).
Figure 78-4
Supraventricular tachycardia at a rate of 180 beats/min
is present; the origin of the tachycardia is not evident (A).
After injection of 6 mg adenosine, atrial flutter waves are clearly visible, thereby
establishing the diagnosis (B).
Bronchospasm has also been described after the injection of adenosine, including intraoperatively.[123] [124] [125] [126] This complication has occurred in patients with bronchial asthma or chronic obstructive pulmonary disease (COPD). The mechanism is unknown, but the bronchospasm may result from stimulation of bronchial smooth muscle adenosine receptors or stimulation of mast cell-derived mediators of bronchoconstriction. Aminophylline, an adenosine receptor antagonist, has been used successfully intraoperatively to treat adenosine-induced bronchospasm.[126] In light of this experience, adenosine should not be used or should be used with caution in patients with a history of
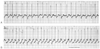 Figure 78-5
A, Atrial fibrillation
with a ventricular response of 190 beats/min. B,
After the injection of 6 mg adenosine, the ventricular response increased to 240
beats/min. (From White RD: Acceleration of the ventricular response in
paroxysmal lone atrial fibrillation following the injection of adenosine. Am J Emerg
Med 11:245, 1993.)
Figure 78-5
A, Atrial fibrillation
with a ventricular response of 190 beats/min. B,
After the injection of 6 mg adenosine, the ventricular response increased to 240
beats/min. (From White RD: Acceleration of the ventricular response in
paroxysmal lone atrial fibrillation following the injection of adenosine. Am J Emerg
Med 11:245, 1993.)
If the PSVT does not respond to adenosine or if it recurs, verapamil is the drug of choice. A dose of 5 mg produces a more sustained block of AV nodal conduction. Verapamil can also be used to slow the ventricular response in atrial fibrillation or flutter.[127] However, it should not be used in patients with Wolff-Parkinson-White syndrome in whom atrial fibrillation or flutter develops. In this setting, verapamil-induced increases in conduction over the accessory pathway may produce alarming acceleration of the ventricular rate or VF.[128] [129] [130] If atrial flutter or fibrillation results in hemodynamic deterioration because of the rapid
 Figure 78-6
The cardiac adenosine system is composed of three components:
(1) formation, (2) receptor complex effects, and (3) degradation. (1) Adenosine
(ADO) can be formed intracellularly through the adenosine triphosphate (ATP) or S-adenosylhomocysteine
(SAH) pathway or extracellularly by breakdown of adenosine nucleotides. (2) The
adenosine receptor (ADO-R) is coupled to ion channels by means of guanine-binding
regulatory proteins (G i). Theophylline (THEO) derivatives act as competitive antagonists
for adenosine receptors. (3) ADO can be transported into the cell and then degraded
by deamination to inosine or phosphorylated to adenosine monophosphate (AMP). Dipyridamole
can block the cellular uptake of ADO, thus prolonging its effect. ADP, adenosine
diphosphate, cAMP, cyclic AMP; GTP, guanosine triphosphate. (Redrawn with
modification from Bertolet BD, Hill JA: Adenosine: Diagnostic and therapeutic uses
in cardiovascular medicine. Chest 104:1860, 1993.)
Figure 78-6
The cardiac adenosine system is composed of three components:
(1) formation, (2) receptor complex effects, and (3) degradation. (1) Adenosine
(ADO) can be formed intracellularly through the adenosine triphosphate (ATP) or S-adenosylhomocysteine
(SAH) pathway or extracellularly by breakdown of adenosine nucleotides. (2) The
adenosine receptor (ADO-R) is coupled to ion channels by means of guanine-binding
regulatory proteins (G i). Theophylline (THEO) derivatives act as competitive antagonists
for adenosine receptors. (3) ADO can be transported into the cell and then degraded
by deamination to inosine or phosphorylated to adenosine monophosphate (AMP). Dipyridamole
can block the cellular uptake of ADO, thus prolonging its effect. ADP, adenosine
diphosphate, cAMP, cyclic AMP; GTP, guanosine triphosphate. (Redrawn with
modification from Bertolet BD, Hill JA: Adenosine: Diagnostic and therapeutic uses
in cardiovascular medicine. Chest 104:1860, 1993.)
Atrial flutter or fibrillation with a rapid ventricular response can cause rate-related hemodynamic compromise manifested perioperatively as hypotension or decreased cardiac output (or both). In hemodynamically unstable patients, cardioversion should be used. Recent-onset atrial flutter is typically very sensitive to low-energy shocks (e.g., 50 J) ( Fig. 78-7 ). Atrial flutter can also be terminated by overdrive pacing with external generators that have this capability. For atrial fibrillation, the initial energy dose should be 100 J and increased as needed to 200, 300, and 360 J.
| Peripheral (antecubital) | 6 mg, then 12 mg if needed |
| Central | 3 mg, then 6 mg if needed |
| If taking theophylline-containing drugs | 9 mg peripherally, 6 mg centrally |
| If taking dipyridamole | 2 mg peripherally, 1 mg centrally |
 Figure 78-7
Synchronized cardioversion algorithm. PSVT, paroxysmal
supraventricular tachycardia; VT, ventricular tachycardia. (Redrawn with
modification from American Heart Association in collaboration with International
Liaison Committee on Resuscitation: Guidelines 2000 for Cardiopulmonary Resuscitation
and Emergency Cardiovascular Care: International Consensus on Science, Parts 1–12.
Circulation 102(Suppl I):I–1, 2000.)
Figure 78-7
Synchronized cardioversion algorithm. PSVT, paroxysmal
supraventricular tachycardia; VT, ventricular tachycardia. (Redrawn with
modification from American Heart Association in collaboration with International
Liaison Committee on Resuscitation: Guidelines 2000 for Cardiopulmonary Resuscitation
and Emergency Cardiovascular Care: International Consensus on Science, Parts 1–12.
Circulation 102(Suppl I):I–1, 2000.)
In hemodynamically stable patients with rapid ventricular rates secondary to atrial fibrillation or flutter, treatment is pharmacologic. With acute onset of these tachyarrhythmias, ibutilide given intravenously has the most rapid onset of effect in restoring sinus rhythm.[131] [132] Ibutilide is a class III antiarrhythmic drug that prolongs the action potential duration and effective refractory period without effects on the action potential upstroke. The dose is 1 mg given over a 10-minute period. A second dose can be administered 10 minutes after the first, if necessary. Conversion to sinus rhythm is more frequent in atrial flutter than in atrial fibrillation (63% versus 31%) and more frequent in atrial fibrillation of shorter duration.[131] Prolongation of the QT interval reflects the pharmacologic action of the drug: polymorphic ventricular tachycardia (PVT) accompanied by increases in the
 Figure 78-8
Multifocal atrial tachycardia. P waves with several
different morphologic identities are evident.
Figure 78-8
Multifocal atrial tachycardia. P waves with several
different morphologic identities are evident.
Alternative options for the treatment of supraventricular arrhythmias include diltiazem, verapamil, β-blocking medications, procainamide, and amiodarone. By slowing conduction and increasing refractoriness in the AV node, calcium channel blocking agents result in ventricular rate control in atrial flutter, atrial fibrillation, and multifocal atrial tachycardia. Diltiazem is given as a loading dose of 0.25 mg/kg over a period of 2 minutes, followed, if needed, in 10 to 15 minutes by 0.35 mg/kg. An infusion at a rate of 5 to 15 mg/hr can be used to maintain rate control. If verapamil is used, 5 mg can be given initially and then 5 to 10 mg in 15 to 30 minutes if needed. β-Blocking medications are helpful in ventricular rate control when no contraindications to use are present. Amiodarone is a complex agent with antiadrenergic effects in the presence of supraventricular arrhythmias and tachycardias resulting from accessory pathways or rapid AV node transmission of atrial impulses and in situations in which other agents have failed to control the heart rate. It is given as a 150-mg intravenous bolus over a 10-minute period, followed by a 1-mg/min infusion for 6 hours to a maximum daily dose of 2 g.
Multifocal (multiform) atrial tachycardia is a quite common tachyarrhythmia that is often misdiagnosed as atrial fibrillation.[133] [134] [135] Increased automaticity in multiple atrial foci results in a need for therapy different from that for reentrant supraventricular arrhythmias (atrial flutter, atrial fibrillation, PSVT). Multifocal atrial tachycardia is diagnosed by observing the presence of at least three morphologically different P waves in the same lead of a 12-lead electrocardiogram (ECG) and a ventricular rate greater than 100/min ( Fig. 78-8 ). It is usually described as occurring in patients with COPD, especially during exacerbations, and ICU management is necessary. However, it occurs in other settings as well, such as hypokalemia, catecholamine administration, and acute
 Figure 78-9
Slow (14 beats/min) idioventricular escape rhythm in
a patient with carotid sinus hypersensitivity. External or transvenous pacing is
the treatment of choice for bradyarrhythmias such as this.
Figure 78-9
Slow (14 beats/min) idioventricular escape rhythm in
a patient with carotid sinus hypersensitivity. External or transvenous pacing is
the treatment of choice for bradyarrhythmias such as this.
In the setting of emergency cardiovascular care, the brady-arrhythmia arising within the ventricles that is in need of urgent treatment is complete heart block with a very slow idioventricular escape rhythm (e.g., 15 to 30 beats/min) ( Fig. 78-9 ). In this situation, atropine can be tried, but the treatment of choice is external or transvenous pacing as soon as it can be accomplished. If an external pacemaker is available, pacing should be instituted quickly.
In the category of potentially life-threatening, and sometime prearrest, arrhythmias, VT ( Fig. 78-10 ) is commonly in need of urgent intervention. VT that produces cardiac arrest (pulseless VT) is discussed with VF. Treatable causes of VT should always be sought before or during pharmacologic or electrical interventions. Hypoxemia, hypercapnia, hypokalemia or hypomagnesemia (or both), digitalis toxicity, and acid-base derangements are obvious considerations.
Treatment of VT is dependent on the degree of hemodynamic compromise induced by the tachycardia. If the patient remains stable and ventricular function is preserved (no evidence of pulmonary edema or a known history of left ventricular function), procainamide and synchronized cardioversion are the treatment choices. If cardioversion is chosen, the energy levels are identical to those used in unstable patients. If procainamide is chosen, a loading
 Figure 78-10
Ventricular tachycardia. The rate is 280 beats/min,
the QRS width is 160 msec, and three consecutive capture/fusion beats are seen.
Figure 78-10
Ventricular tachycardia. The rate is 280 beats/min,
the QRS width is 160 msec, and three consecutive capture/fusion beats are seen.
In unstable patients (e.g., in the presence of systemic hypotension, pulmonary edema, or clinical or ECG signs of acute ischemia or infarction), synchronized cardioversion is the treatment of choice with either monophasic energy doses of 100, 200, 300, and 360 J or biphasic, nonescalating, impedance-compensated energy doses of 150 J in rapid succession as indicated. An algorithm for cardioversion of a variety of tachyarrhythmias, including VT, is shown in Figure 78-7 . It must be clear that in the ACLS arrhythmia treatment algorithms, "equivalent biphasic" energies refers to nonescalating, impedance-compensated biphasic 150-J shocks.
An atypical form of VT known as torsades de pointes ("twisting of the points") that was originally described in 1966 is characterized by twisting of the QRS axis around the baseline and a polymorphic appearance.[142] [143] [144] [145] [146] The underlying electrophysiologic derangement in this arrhythmia is a marked and nonuniform delay in repolarization manifested as a prolonged QT interval on the ECG. It typically begins with a characteristic long-short initiating sequence ( Fig. 78-11 ). It can be induced by drugs such as quinidine, procainamide, disopyramide, and phenothiazine and also by bradycardia, hypokalemia, hypomagnesemia, or acute ischemia or infarction.[147] Even though amiodarone produces marked prolongation of repolarization, it rarely causes torsades de pointes, possibly because the drug-induced prolonged repolarization is
 Figure 78-11
Torsades de pointes ventricular tachycardia. The QTc
interval is 450 msec during the sinus rhythm preceding the tachycardia. This arrhythmia
occurred in a patient with acute myocardial infarction.
Figure 78-11
Torsades de pointes ventricular tachycardia. The QTc
interval is 450 msec during the sinus rhythm preceding the tachycardia. This arrhythmia
occurred in a patient with acute myocardial infarction.
If PVT is present (i.e., without evident QT prolongation), standard antiarrhythmic therapy as outlined earlier for VT can be tried, although magnesium sulfate should be considered early in the treatment of this form of PVT as well, particularly if torsades de pointes is suspected by history but QT prolongation cannot be confirmed on the ECG. In fact, a 12-lead ECG is often needed to identify QT lengthening. If hemodynamic deterioration occurs with PVT, defibrillation should be used as the initial treatment.
An algorithm for the treatment of tachyarrhythmias that encompasses those of both supraventricular and ventricular origin is shown in Figure 78-13 . This algorithm makes provision for intervention in patients with a wide-QRS complex tachycardia ( Fig. 78-14 ) of indeterminate origin based on the patient's hemodynamic stability. In a hemodynamically stable patient, Guidelines 2000 emphasizes making a specific rhythm diagnosis and identifying the presence of clinically significant reductions in ventricular function rather than attempting a diagnosis based on response to antiarrhythmic medications.[157]
 Figure 78-12
Polymorphic ventricular tachycardia. The QRS complexes
have varying morphology, but without the "twisting of the points" torsades morphology
seen in Figure 78-11
. The
tachycardia terminated spontaneously. (From White RD, Wood DL: Out-of-hospital
pleomorphic ventricular tachycardia and resuscitation: Association with acute myocardial
ischemia and infarction. Ann Emerg Med 21:1282, 1992.)
Figure 78-12
Polymorphic ventricular tachycardia. The QRS complexes
have varying morphology, but without the "twisting of the points" torsades morphology
seen in Figure 78-11
. The
tachycardia terminated spontaneously. (From White RD, Wood DL: Out-of-hospital
pleomorphic ventricular tachycardia and resuscitation: Association with acute myocardial
ischemia and infarction. Ann Emerg Med 21:1282, 1992.)
Verapamil should be avoided in any wide-QRS complex tachycardia unless it is known with certainty that the tachycardia is supraventricular in origin; if VT is present, verapamil can induce severe hypotension or cardiovascular collapse in as many as 87% of such misdiagnosed episodes ( Fig. 78-15 ). Any wide-complex tachycardia of uncertain origin (despite careful scrutiny of the ECG for all helpful clues) should be assumed to be VT and treated accordingly. If the patient is hemodynamically deteriorating, cardioversion should be used initially without a previous trial of drug therapy.
|
|
|
|
|
|
|
|
|
|
|
|
|