|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A large study of 18,473 PACU admissions in a university hospital in 1986 to 1989 found the incidence of PACU complications to be nearly 24%.[60] The most common complications were nausea and vomiting (9.8%), need for airway support (6.9%), hypotension (2.7%), dysrhythmia (1.4%), hypertension (1.1%), altered mental status (0.6%), need to rule out myocardial infarction (0.3%), and major cardiac events (0.3%) ( Fig. 71-2 ). Greater ASA physical status, anesthesia duration between 2 and 4 hours, emergency procedures, and abdominal and orthopedic procedures had the highest incidence of complications.
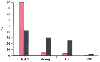 Figure 71-2
Complications in the postanesthesia care unit (PACU).
Two studies examined the frequency of PACU complications. Among inpatients, 24%
of patients had a complication, whereas among outpatients, only 8% of patients had
a complication. Nausea and vomiting (N & V) were the most common in both studies.
Life-threatening cardiovascular (CV) and respiratory complications were rare in
outpatients. Nausea and vomiting accounted for 90% of the complications in outpatients.
CNS, central nervous system. (Red bars—outpatients,
from Duncan PG, Cohen MM, Tweed WA, et al: The Canadian four-centre study of anaesthetic
outcomes. III Are anaesthetic complications predictable in day case practice? Can
J Anaesth 39:440, 1992; black bars—inpatients,
from Hines R, Barash PG, Watrous G, et al: Complications occurring in the postanesthesia
care unit: A survey. Anesth Analg 74:503, 1992.)
Figure 71-2
Complications in the postanesthesia care unit (PACU).
Two studies examined the frequency of PACU complications. Among inpatients, 24%
of patients had a complication, whereas among outpatients, only 8% of patients had
a complication. Nausea and vomiting (N & V) were the most common in both studies.
Life-threatening cardiovascular (CV) and respiratory complications were rare in
outpatients. Nausea and vomiting accounted for 90% of the complications in outpatients.
CNS, central nervous system. (Red bars—outpatients,
from Duncan PG, Cohen MM, Tweed WA, et al: The Canadian four-centre study of anaesthetic
outcomes. III Are anaesthetic complications predictable in day case practice? Can
J Anaesth 39:440, 1992; black bars—inpatients,
from Hines R, Barash PG, Watrous G, et al: Complications occurring in the postanesthesia
care unit: A survey. Anesth Analg 74:503, 1992.)
Another large study of 37,079 patients (excluding cardiac, obstetric, craniotomy, thoracotomy, laparotomy, and emergency operations) recovering from anesthesia in a tertiary care university hospital from July 1992 through June 1997 found that minor anesthesia-related incidents, events, and complications occurred in 22.1% of patients.[61] Severe events occurred in 0.2% of the patients. Even a minor IEC significantly increased PACU utilization.
Complications in an ambulatory surgical PACU are similar but follow a different distribution. Duncan and colleagues[62] found that because cardiac and respiratory events were infrequent, nausea and vomiting emerged as the major complication in outpatients (see Fig. 71-2 ).
Nearly two thirds of major anesthesia-related PACU incidents may be respiratory.[63] The major respiratory
 Figure 71-3
Soft tissue roentgenograms of the head and neck of an
awake supine patient show that with the head in the neutral position, air clearance
at the base of the tongue is about 3 mm. When the patient is unconscious, this small
opening is often lost and airway obstruction results. (From Ruben HM, Elam
JO, Ruben AM, et al: Investigation of upper airway problems in resuscitation. Anesthesiology
22:271, 1961.)
Figure 71-3
Soft tissue roentgenograms of the head and neck of an
awake supine patient show that with the head in the neutral position, air clearance
at the base of the tongue is about 3 mm. When the patient is unconscious, this small
opening is often lost and airway obstruction results. (From Ruben HM, Elam
JO, Ruben AM, et al: Investigation of upper airway problems in resuscitation. Anesthesiology
22:271, 1961.)
In an evaluation of 24,157 consecutive PACU admissions over a 33-month period, Rose and associates[64] found that for patients receiving general anesthesia, the risk of a critical respiratory event was 1.3% (hypoxemia, 0.9%; hypoventilation, 0.2%; and airway obstruction, 0.2%). Risk factors were age older than 60 years, male gender, diabetes, obesity, emergencies, surgery longer than 4 hours, opioid or sedative premedication, and the use of thiopental as opposed to propofol. Patients who did have a critical respiratory event had longer PACU stays and more cardiac problems and were more likely to require ICU admission.
The most common cause of postoperative airway obstruction is pharyngeal obstruction ( Fig. 71-3 ). [65] A combination of backward tilt of the head and anterior displacement of the mandible is often helpful ( Fig. 71-4 and Fig. 71-5 ). The proper position for each patient will depend on trial and error until a patent airway is obtained. If the obstruction is not immediately reversible, a nasal or oral airway can be inserted. Patients may better tolerate the nasal airway. The oral airway may stimulate gagging and vomiting, as well as laryngeal spasm.
Laryngeal obstruction can also occur secondary to laryngeal spasm, direct airway injury, or even vocal cord paralysis.[66] [67] [68] If the airway obstruction is due to laryngeal spasm, it can sometimes be relieved by anterior displacement of the mandible.[69] If the obstruction cannot be relieved by simple maneuvers, 10 mg dexamethasone intravenously may reopen the airway.
All patients with airway obstruction should receive oxygen by facemask (FIO2 of 1.0). When the airway cannot be opened by physical means, positive-pressure ventilation with a bag, mask, and 100% oxygen is indicated. If succinylcholine has been given, assisted ventilation should be continued for at least 5 to 10 minutes, even if the obstruction has been relieved.[70] For all cases of airway obstruction, if an adequate airway cannot be established by simple physical or pharmacologic means, orotracheal intubation is necessary.
The laryngeal mask airway may be helpful in certain patients and has even been used to provide pressure support ventilation in the PACU.[71] In the very rare case in which the trachea cannot be intubated, an emergency
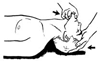 Figure 71-4
A combination of backward head tilt and anterior mandibular
displacement is most effective in relieving airway obstruction from pharyngeal blockade
by the tongue. This maneuver will also be helpful in relieving obstruction caused
by laryngeal spasm. (From Ruben HM, Elam JO, Ruben AM, et al: Investigation
of upper airway problems in resuscitation. Anesthesiology 22:271, 1961.)
Figure 71-4
A combination of backward head tilt and anterior mandibular
displacement is most effective in relieving airway obstruction from pharyngeal blockade
by the tongue. This maneuver will also be helpful in relieving obstruction caused
by laryngeal spasm. (From Ruben HM, Elam JO, Ruben AM, et al: Investigation
of upper airway problems in resuscitation. Anesthesiology 22:271, 1961.)
Patients with obstructive sleep apnea are at high risk for airway obstruction when sedated. Nasal continuous positive airway pressure (CPAP) can be very useful in these patients after tracheal extubation.[72]
After major surgical procedures, all patients should receive oxygen therapy by facemask or nasal prongs. Healthy patients may not require oxygen therapy after a brief minor surgical procedure. The need for such therapy can be guided by pulse oximetry.[73] [74]
Two different oxygen delivery systems were studied in healthy outpatients.[75]
 Figure 71-5
Radiographs of the head and neck of an anesthetized patient
show that combining backward head tilt with anterior mandibular displacement results
in increased clearance at the tongue base to 22 mm (see Fig.
71-3
). (From Ruben HM, Elam JO, Ruben AM, et al: Investigation
of upper airway problems in resuscitation. Anesthesiology 22:271, 1961.)
Figure 71-5
Radiographs of the head and neck of an anesthetized patient
show that combining backward head tilt with anterior mandibular displacement results
in increased clearance at the tongue base to 22 mm (see Fig.
71-3
). (From Ruben HM, Elam JO, Ruben AM, et al: Investigation
of upper airway problems in resuscitation. Anesthesiology 22:271, 1961.)
In a different study, 293 postsurgical patients who had not undergone thoracic, upper abdominal, or neurologic surgery were randomly assigned in the PACU to receive[76]
A randomized, blinded study of the effects of oximetry found that episodes of extreme hypoxemia (SaO2 < 80%) were not seen in patients for whom the PACU staff had access to the pulse oximetry data but that such episodes were encountered when they did not. Interestingly, the overall complication rates in the two groups were not different.[77]
Hypoxemia after anesthesia and surgery can be caused by many factors. Evaluation of hypoxemic postoperative patients should include consideration of each of the classic causes of hypoxemia:
Low inspired concentrations of oxygen (FIO2 < 0.21) are rare causes of significant postoperative hypoxemia; however, delivery of hypoxic gas mixtures to postoperative patients is possible. Crossing of nitrous oxide and oxygen pipelines during hospital construction resulted in the loss of more than 30 lives in a Canadian hospital. Pipelines have crossed in hospital modernization projects; switching of all adapters can also lead to delivery of the wrong gas, as can purging the system with nitrogen for pipeline repairs (also see Chapter 9 ).[80]
The most common cause of postoperative hypoxemia is an increase in right-to-left intrapulmonary shunting. Atelectasis (i.e., collapse of an entire lung, lobe, or lung segment) is the most common cause of an increased right-to-left shunt. Bronchial obstruction from secretions or blood is a frequent cause of atelectasis. Lobar and segmental collapse often results from bronchial obstruction with secretions and is best managed by providing adequate humidification of inspired gases, coughing, deep breathing, and postural drainage.
Pneumothorax is another potential cause of hypoxemia in the PACU. Pneumothorax causes hypoxemia secondary to atelectasis and intrapulmonary shunting. Pneumothorax occurs as a result of direct lung or airway injury from trauma, rib fractures, or attempts at percutaneous vascular cannulation. Pneumothorax resulting from mechanical ventilation per se is rare unless airway pressure is high.[81] [82]
Treatment depends on the size of the pneumothorax and the patient's condition. A 10% to 20% pneumothorax in a spontaneously breathing patient can be observed with frequent upright chest roentgenograms. A pneumothorax of more than 20% in a spontaneously breathing patient or any pneumothorax in a mechanically ventilated patient should be treated by insertion of a chest tube for drainage, usually in the second intercostal space in the midclavicular line.
Tension pneumothorax leads to circulatory compromise as a result of the pleural cavity filling with air and compressing the mediastinum. A 14-gauge needle inserted into the second intercostal space can relieve the tension before chest tube insertion.
Arterial hypoxemia may be present in postoperative patients who have no discernible change in their chest radiogram. These patients may have an increased right-to-left intrapulmonary shunt as a result of diffuse airway collapse. The relationship between the functional residual capacity (FRC) of the lung and closing capacity is a prime determinant of this effect.[83] [84]
When closing capacity exceeds FRC, airways collapse during tidal breathing and an intrapulmonary shunt develops. Any situation that results in either increased closing capacity (e.g., increasing age) or reduced FRC (pulmonary edema, infection, aspiration, obesity) will place the patient at increased risk for hypoxemia.
Pulmonary edema can also be a cause of hypoxemia in the postoperative period. Although pulmonary edema has been well studied in patients with left ventricular failure, studies of this process in the immediate postoperative period are rare. Cooperman and Price examined 40 cases of perioperative pulmonary edema and found half the patients to have preoperative evidence of cardiovascular disease ( Fig. 71-6 ).[85] The most common time of appearance of pulmonary edema was within 60 minutes of completion of surgery. More than half these cases were preceded by hypertension, thus suggesting that this problem may be related to the high pulmonary vascular pressure seen in acute, postoperative hypertension. Pulmonary edema was frequently detected by the presence of wheezing.
Prolonged airway obstruction can cause "negative-pressure" pulmonary edema.[86] [87] Current treatment of both forms of pulmonary edema involves lowering hydrostatic pressure in the lungs to the lowest possible level
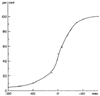 Figure 71-6
In a study of 40 cases of postoperative pulmonary edema,
the cumulative incidence of pulmonary edema (percentage) is plotted with respect
to the end of the operation (minutes). Most cases occurred within the first 30 minutes,
and most were preceded by hypertension. (From Cooperman LH, Price HR: Pulmonary
edema in the operative and postoperative period: Review of 40 cases. Ann Surg 172:883,
1970.)
Figure 71-6
In a study of 40 cases of postoperative pulmonary edema,
the cumulative incidence of pulmonary edema (percentage) is plotted with respect
to the end of the operation (minutes). Most cases occurred within the first 30 minutes,
and most were preceded by hypertension. (From Cooperman LH, Price HR: Pulmonary
edema in the operative and postoperative period: Review of 40 cases. Ann Surg 172:883,
1970.)
Positive-pressure ventilation is useful in patients with severe hypoxemia or respiratory acidosis. Ventilation with end-expiratory pressure improves oxygenation by increasing lung volume, not by decreasing lung water.[88]
Pulmonary embolism occurring in the immediate postoperative period is a serious event that can lead to profound hypoxemia. Patients at bed rest for prolonged periods before surgery, patients who have undergone joint replacement surgery, or parturients are particularly susceptible to emboli. The diagnosis is suspected in a patient with sudden pleuritic chest pain, shortness of breath, pleural effusion, or tachypnea. Massive emboli result in hypotension, pulmonary hypertension, and elevated central venous pressure. Because the treatment of choice is anticoagulation, establishment of an accurate diagnosis is imperative so that patients in the immediate postsurgical period are not needlessly exposed to the risks of anticoagulation.
Posthyperventilation hypoxemia and diffusion hypoxemia can occur but are rarely seen in clinical practice because oxygen administration prevents manifestation of these conditions. Diffusion hypoxia occurs when N2 O is replaced with air at the end of the anesthetic. Because N2 O is 31 times more soluble than nitrogen, inspired air is diluted with N2 O and PAO2 falls. The combination of hyperventilation and N2 O can result in the drop in PAO2 seen in the bottom line of Figure 71-7 .[89]
The type of anesthetic and the site of surgery influence the reduction in arterial oxygen tension (PaO2 ) seen after anesthesia and surgery. Abdominal operations are associated with the most prolonged reductions in PaO2 . A shift
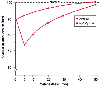 Figure 71-7
Posthyperventilation hypoxia and diffusion hypoxia.
The upper (dashed) line represents normal PAO2
during breathing of air. Posthyperventilation hypoxia is indicated by the upper
solid line, assuming that the patient hyperventilated for an hour and
was then allowed to accumulate CO2
for 10 minutes before zero time. Diffusion
hypoxia (after equilibration with 79% N2
O) was added to posthyperventilation
hypoxia as above, except that the inspired gas was changed to air at zero time.
(Redrawn from Marshall BE, Wyche MQ: Hypoxemia during and after anesthesia.
Anesthesiology 37:178, 1972.)
Figure 71-7
Posthyperventilation hypoxia and diffusion hypoxia.
The upper (dashed) line represents normal PAO2
during breathing of air. Posthyperventilation hypoxia is indicated by the upper
solid line, assuming that the patient hyperventilated for an hour and
was then allowed to accumulate CO2
for 10 minutes before zero time. Diffusion
hypoxia (after equilibration with 79% N2
O) was added to posthyperventilation
hypoxia as above, except that the inspired gas was changed to air at zero time.
(Redrawn from Marshall BE, Wyche MQ: Hypoxemia during and after anesthesia.
Anesthesiology 37:178, 1972.)
Reductions in cardiac output can contribute to large decreases in PaO2 in patients with existing intrapulmonary shunts because of the effect of the lowered mixed venous PO2 , which is added directly to the arterial circulation through the right-to-left shunt ( Fig. 71-8 ).[89] Postoperative shivering can result in increases in oxygen consumption by as much as 500%; however, such shivering only rarely contributes to arterial hypoxemia.[79]
Treatment of hypoxemia by facemask oxygen is effective in restoring PaO2 in many cases. The PaO2 response to oxygen breathing depends on the degree of intrapulmonary shunting. Increasing the FIO2 from room air to 100% results in a large increase in PaO2 when the shunt fraction is small; however, oxygen will have little effect on PaO2 in patients with a large shunt fraction ( Fig. 71-9 ).[91] If hypoxemia persists (PaO2 < 60 mm Hg) despite maximal oxygen therapy (FIO2 = 1.0), tracheal intubation and mechanical ventilation should be initiated. In such patients, ventilation with PEEP will increase FRC and result in an improvement in arterial oxygenation. Ventilation with PEEP eventually permits a reduction in FIO2 without a fall in PaO2 .
The use of CPAP by an external mask (mask or nasal CPAP) is increasingly being used for the treatment of patients with severe hypoxemia who have adequate carbon dioxide elimination (also see Chapter 75 ).[92] Good candidates for mask or nasal CPAP are patients with severe hypoxemia requiring more than 80% oxygen to achieve
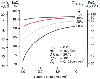 Figure 71-8
Relationship between cardiac index and arterial oxygenation
with shunt fractions (QVA
/QT
) ranging from 5% to 30%. With
large intrapulmonary shunts, a small drop in cardiac index results in a large fall
in arterial oxygenation. (Redrawn from Philbin DM, Sullivan SF, Bowman FO,
et al: Postoperative hypoxemia. Contribution of cardiac output. Anesthesiology
32:136, 1970.)
Figure 71-8
Relationship between cardiac index and arterial oxygenation
with shunt fractions (QVA
/QT
) ranging from 5% to 30%. With
large intrapulmonary shunts, a small drop in cardiac index results in a large fall
in arterial oxygenation. (Redrawn from Philbin DM, Sullivan SF, Bowman FO,
et al: Postoperative hypoxemia. Contribution of cardiac output. Anesthesiology
32:136, 1970.)
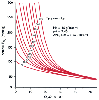 Figure 71-9
Relationship between shunt fraction and arterial oxygen
tension for varying inspired concentrations of oxygen (PaO2
,
100 to 680). Patients with small shunt fractions have large increases in PaO2
with increasing inspired oxygen, whereas patients with high shunt fractions have
a small response to increasing oxygen concentration. (Redrawn from Pontoppidan
H, Layer MB, Geffin B: Acute respiratory failure in the surgical patient. Adv Surg
4:163, 1970.
Figure 71-9
Relationship between shunt fraction and arterial oxygen
tension for varying inspired concentrations of oxygen (PaO2
,
100 to 680). Patients with small shunt fractions have large increases in PaO2
with increasing inspired oxygen, whereas patients with high shunt fractions have
a small response to increasing oxygen concentration. (Redrawn from Pontoppidan
H, Layer MB, Geffin B: Acute respiratory failure in the surgical patient. Adv Surg
4:163, 1970.
Hypoventilation is defined as reduced alveolar ventilation resulting in an increase in arterial carbon dioxide tension (PaCO2 ). During the postoperative period, hypoventilation occurs as a result of poor respiratory drive, poor respiratory muscle function, or a high rate of production of carbon dioxide, or it can be a direct result of acute or chronic lung disease.
Central respiratory depression is seen with any anesthetic. Narcotic anesthetics produce respiratory depression that is detectable by a shift of the carbon dioxide response curve downward and to the right (also see Chapter 11 ).
Narcotic-induced respiratory depression can be reversed with the use of narcotic antagonists.[93] When small doses are used, these agents can reverse the narcotic-induced respiratory depression without altering pain relief; however, the duration of action of the antagonists currently available is shorter than that of most narcotics, and the dose has to be repeated at least once. Titration of a small dose and increasing the dose upward until an effect is seen can avoid the sudden onset of severe pain along with the profound reflex tachycardia and hypertension. These increases in rate-pressure product (heart rate × systolic blood pressure) signify large increases in myocardial oxygen consumption and can result in ischemia in patients with coronary artery disease.
Poor respiratory muscle function after surgery can contribute to hypoventilation. The site of the incision affects the ability to take a large breath as measured by vital capacity. Nearly all patients have reductions in vital capacity that are the greatest on the day of surgery. Patients undergoing upper abdominal surgery have the greatest reduction in vital capacity, with as much as a 60% reduction on the day of surgery ( Fig. 71-10 ).[94] This reduced vital capacity, which is secondary to diaphragmatic impairment, results in problems in both carbon dioxide elimination and oxygenation.[90]
Failure of reversal of neuromuscular blocking drugs may result in inadequate respiratory muscle function postoperatively. Such failure can be due to inadequate excretion of the drug, as in renal failure, or the presence of other drugs that attenuate neuromuscular blockade, such as gentamicin, neomycin, clindamycin, or furosemide (also see Chapter 13 ). [95] [96] Hypermagnesemia potentates neuromuscular blockade, as does hypothermia.[97]
Obesity, gastric dilation, tight dressings, and body casts also inhibit respiratory muscle function and can predispose to CO2 retention. Measurement of PaCO2 is the best method of detecting hypoventilation in the postoperative period. Although hypertension and tachycardia commonly occur during CO2 retention, they may not be seen in postsurgical patients and the elderly, who have an attenuated response to increased levels of CO2 (also see Chapter 62 ).
Measurement of vital capacity and maximum inspiratory force is a good guide to the ability of a postsurgical
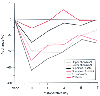 Figure 71-10
Change in vital capacity seen after surgical procedures
in various sites. All patients manifest the lowest vital capacity on the day of
surgery. Upper abdominal surgery is associated with the greatest reduction in vital
capacity, and spinal surgery is associated with the least. (Redrawn from
Ali J, Weisel RD, Layug AB, et al: Consequences of postoperative alterations in
respiratory mechanics. Am J Surg 128:376, 1974.)
Figure 71-10
Change in vital capacity seen after surgical procedures
in various sites. All patients manifest the lowest vital capacity on the day of
surgery. Upper abdominal surgery is associated with the greatest reduction in vital
capacity, and spinal surgery is associated with the least. (Redrawn from
Ali J, Weisel RD, Layug AB, et al: Consequences of postoperative alterations in
respiratory mechanics. Am J Surg 128:376, 1974.)
Treatment of serious respiratory failure necessitates emergency tracheal intubation. The need to perform reintubation is rare and occurred in 0.2% of about 13,000 patients studied.[99] In that study, 77% of the reintubations occurred within the first hour in the PACU and were more common in children and the elderly. Many of the cases were thought to be preventable and related to excessive anesthetic and sedative drugs, excessive fluid administration, persistent effects of muscle relaxants, and upper airway obstruction.
|
|
|
|
|
|
|
|
|
|
|
|
|