|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Critical cardiovascular events are the second major group of life-threatening complications for patients in the PACU. Rose and coworkers[100] studied 18,380 patients after general anesthesia in Toronto and found that patients in whom hypertension or tachycardia developed in the PACU had more unplanned critical care admissions and a higher mortality rate than did those in whom these conditions did not develop. In contrast to respiratory events, anesthetic factors contributed in only a minor way to the development of cardiovascular problems in the PACU; patient and surgical risk factors were more important.
The recovery phase of anesthesia is usually associated with decreased ventricular preload, reduced myocardial contractility, or a profound reduction in systemic vascular resistance. Decreased ventricular preload is caused by intravascular volume depletion because of blood loss, excessive third-space fluid loss, unreplaced urinary losses, or septicemia with vasodilatation and capillary leakage of fluid (also see Chapter 47 , Chapter 48 , and Chapter 63 ).
Acute, massive pulmonary embolism produces hypotension by blocking the flow of blood to the left side of the heart. Reductions in myocardial contractility occur because of the continued effects of anesthetic drugs, preexisting ventricular dysfunction, or the development of perioperative myocardial infarction. Profound reductions in systemic vascular resistance usually occur with septicemia, but they are also seen in chronic liver failure.
Prompt diagnosis and treatment are important because prolonged hypotension can result in hypoperfusion of vital organs and subsequent ischemic damage. If hypotension persists despite attempts to restore intravascular volume, ventricular preload must be further assessed. In patients with normal left ventricular function, central venous pressure will estimate ventricular preload.
In the presence of left ventricular dysfunction, central venous pressure will not be an accurate guide to ventricular filling pressure, and a flow-directed pulmonary artery catheter should be considered (also see Chapter 32 ).[100] [101] During this time, administration of a vasopressor will prevent a prolonged period of hypotension while hemodynamic monitoring is established. Ventricular preload can be assessed by measuring pulmonary artery occlusion pressure (PAOP). Bedside measurement of cardiac output is also possible.
Hypovolemic shock is characterized
by low PAOP (<5 to 10 mm Hg) with a normal low cardiac index (normal, 2.5 to 4.0
L/min/m2
) and normal or elevated systemic vascular resistance:
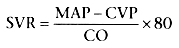
Normal is 900 to 1400 dynes/sec/cm-5
; SVR is systemic vascular resistance,
MAP is mean arterial pressure, CVP is central venous pressure, and CO is cardiac
output (also see Chapter 16
and Chapter 31
).
Cardiogenic shock is characterized by increased PAOP (>15 mm Hg) with a low cardiac index and elevated systemic vascular resistance. Patients in whom left ventricular failure is suspected should have an electrocardiogram and analysis of cardiac enzymes, especially fractionated creatinine phosphokinase to rule out myocardial infarction. Examination of total creatinine phosphokinase alone is of no use in a surgical patient because it will frequently be elevated secondary to skeletal muscle damage.
In septic shock, PAOP will usually be low with a very high cardiac output and low systemic vascular resistance. The patient often has fever, an elevated white blood cell count, and some other sign of systemic infection.
Treatment of such prolonged hypotension is now guided by following the variables of ventricular preload, cardiac output, and urinary output. Hypovolemic shock is treated by intravenous administration of blood and crystalloid. The role of albumin replacement in this setting is controversial, but it is widely used (also see Chapter 47 and Chapter 48 ). Albumin is no more effective than crystalloid in restoring intravascular volume and can cause deterioration in renal function when used for hypovolemic resuscitation. [102] Albumin is also far more expensive than crystalloid.
Comparison of the use of albumin versus crystalloid for hypovolemia shows that the only difference in the two groups of patients was more costly resuscitation in the albumin-resuscitated group.[103] Starch solutions are a less expensive and effective alternative to albumin and saline.[104] When lung injury exists, resuscitation with crystalloid, albumin, or starch solutions will not further damage the lung if resuscitation is not performed with increased pulmonary hydrostatic pressure.[105]
Cardiogenic shock is managed by first optimizing ventricular preload.
We therefore attempt to give fluid or blood intravenously to increase preload and
monitor cardiac output and stroke volume (SV):
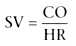
where HR is heart rate. Most patients have optimal cardiac output when PAOP is increased
to 15 to 20 mm Hg (also see Chapter
18
and Chapter 32
).
Occasional patients with severe, long-standing ventricular failure will require a PAOP of 20 to 25 mm Hg to maintain cardiac output. In addition to an optimal preload, these patients also require inotropic support. Some patients with left ventricular failure will still have low cardiac output and high systemic vascular resistance despite optimal preload and inotropic support. In this situation, careful addition of a peripheral vasodilator will lower the outflow impedance to ventricular ejection (vascular resistance is lowered, and stroke volume will increase). Combined inotropic and vasopressor support will often restore cardiac output to nearly normal values ( Fig. 71-11 ). [106]
In this setting, vasodilator therapy should not be abruptly terminated because a rebound rise in vascular resistance often results in worse ventricular function than was present before treatment with the drug was begun[107] (see Chapter 16 for a detailed discussion of these autonomic drugs). Vasodilator therapy should not be used without first increasing preload and increasing the inotropic state of the heart. Vasodilators can be used only in this setting if the hypotension has first been reversed with fluid and inotropic therapy.
Septic shock is managed by replacing the fluid lost from capillary endothelial leak with crystalloid. The use of albumin in this situation is possibly harmful because the albumin can leak out into the interstitium and draw intravascular fluid with it.[108] An inotropic agent is often necessary to further increase cardiac output and raise arterial blood pressure.
On occasion, patients with severe sepsis will remain hypotensive with high cardiac output and lowered vascular resistance despite the infusion of fluids and inotropes. In this situation, prolonged lowered diastolic pressure will result in insufficient coronary blood flow
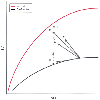 Figure 71-11
The normal Starling relationship of the heart is depicted
on the upper line, where increasing left ventricular
filling pressure (LVFP) increases stroke volume (SV). In the presence of ventricular
dysfunction, this curve is shifted downward and to the right. The depressed curve
can be shifted toward normal by inotropic drugs (I) or vasodilator drugs (V). Infusion
of both (V + I) produces a synergistic effect. Diuretics (D) reduce filling pressure
without increasing output. The dashed line suggests
that SV may rise later. (Redrawn from Cohn JN, Franciosa JA: Vasodilator
therapy of cardiac failure. N Engl J Med 297:27, 1977.)
Figure 71-11
The normal Starling relationship of the heart is depicted
on the upper line, where increasing left ventricular
filling pressure (LVFP) increases stroke volume (SV). In the presence of ventricular
dysfunction, this curve is shifted downward and to the right. The depressed curve
can be shifted toward normal by inotropic drugs (I) or vasodilator drugs (V). Infusion
of both (V + I) produces a synergistic effect. Diuretics (D) reduce filling pressure
without increasing output. The dashed line suggests
that SV may rise later. (Redrawn from Cohn JN, Franciosa JA: Vasodilator
therapy of cardiac failure. N Engl J Med 297:27, 1977.)
When hypertension develops in a patient in the PACU, it is often due to pain, hypercapnia, hypoxemia, urinary retention, or excessive intravascular fluid volume. These etiologies need to be ruled out. Severe hypertension can lead to left ventricular failure, myocardial infarction, or a dysrhythmia as a result of a sharp increase in myocardial oxygen consumption. Acute hypertension may also precipitate acute pulmonary edema or cerebral hemorrhage.
Preexisting hypertension is present in more than half the patients in whom hypertension develops in the recovery room.[109] Such hypertension can be made worse if antihypertensive medications have to be abruptly withdrawn preoperatively.[110] When hypertension does develop during recovery from anesthesia, it usually begins within 30 minutes of the end of the operation ( Fig. 71-12 ).[109]
β-Blocking drugs such as labetalol and esmolol are effective in treating hypertension during recovery. Labetalol, a combined α- and β-blocking agent, is commonly used in the PACU. When used for the treatment of postoperative
 Figure 71-12
Time of onset of hypertension in 60 patients in whom
hypertension developed after surgery. More than half had a history of hypertension.
Pain, emergence excitement, hypercapnia, hypoxemia, and volume overload were common
etiologies. (From Gal TJ, Cooperman LH: Hypertension in the immediate postoperative
period. Br J Anaesth 47:70, 1975.)
Figure 71-12
Time of onset of hypertension in 60 patients in whom
hypertension developed after surgery. More than half had a history of hypertension.
Pain, emergence excitement, hypercapnia, hypoxemia, and volume overload were common
etiologies. (From Gal TJ, Cooperman LH: Hypertension in the immediate postoperative
period. Br J Anaesth 47:70, 1975.)
Factors predisposing to the development of postoperative dysrhythmias are electrolyte imbalance (especially hypokalemia), hypoxia, hypercapnia, metabolic alkalosis and acidosis, and preexisting heart disease (also see Chapter 23 , Chapter 32 , and Chapter 47 ). When a dysrhythmia occurs in a patient in the PACU, it is often a sign of some metabolic or perfusion problem. Dysrhythmias appearing in patients in the PACU rarely need long-term treatment. The most common dysrhythmias are sinus tachycardia, sinus bradycardia, ventricular premature beats, ventricular tachycardia, and supraventricular tachyarrhythmias. These conditions are discussed in Chapter 32 .
|
|
|
|
|
|
|
|
|
|
|
|
|