|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The therapeutic administration of SpO2 or O2 at 1 ATA settings is usually guided by measurement of arterial blood gases. Because elevation of tissue PO2 is usually the major therapeutic aim with the use of HBO, it would be preferable if blood gases could be used in exactly the same way in this setting. However, it is difficult to attain this goal for several practical reasons. First, blood gas values in a sample taken from a patient in a hyperbaric chamber may not remain stable. In any sample of arterial blood, gas has a tendency to diffuse from blood into the small bubbles that inevitably exist within the syringe unless an extremely careful technique is used to remove them. Under normal clinical conditions at 1 ATA, diffusion of O2 and CO2 from blood into a small bubble in a syringe results in only minor changes in blood gas tension. Changes in partial pressure are buffered by the relatively large content of O2 (because of hemoglobin binding) and CO2 (because of the relatively high solubility of CO2 in plasma). However, in blood samples obtained from patients under hyperbaric conditions, large changes in PO2 may result from delay in measurement of an arterial blood sample, changes that are compounded if the measurement is to be made outside the chamber. In this situation, small bubbles present in the syringe before decompression would be correspondingly increased in size after decompression to 1 ATA. Furthermore, at an ambient pressure of 760 mm Hg, the O2 in the blood sample will be supersaturated and tend to move rapidly out of solution. Finally, it is impossible to calibrate blood gas machines accurately for gas tensions higher than ambient barometric pressure. Therefore, measurement of blood gas tension should ideally be performed inside the hyperbaric chamber, which requires a dedicated analyzer and a skilled technician.
If these facilities are not available, two approaches to estimation of PaO2 may be taken. The first is to simply draw the arterial blood sample, extrude the bubbles as carefully as possible, decompress the sample, and measure the sample as quickly as possible. Modern blood gas analyzers can produce a PaO2 estimate by extrapolating the calibration curve obtained from lower O2 tensions. Weaver and Howe[173] have demonstrated that O2 tension in blood samples obtained at 3 ATA in a hyperbaric chamber can be measured accurately at 1 ATA if analysis is performed immediately after decompression of the blood sample.
Another approach is to estimate PaO2 based on measurements obtained at 1 ATA. Gilbert and Keighley[64] demonstrated that at 1 ATA, the ratio of arterial-alveolar PO2 (PaO2 /PAO2 , or the a/A ratio) is relatively constant over a wide range of inspired O2 concentrations. We have examined this ratio over a range of inspired O2 concentrations from 0.21 to 1.0 and ambient pressures from 1 to 3 ATA. Arterial blood gases were obtained at 1 ATA, from which the a/A ratio was calculated. Predicted values for PaO2 were then calculated for breathing air or 100% O2 at elevated ambient pressure and correlated with actual measurements of PaO2 inside the chamber. There was reasonable agreement between predicted and actual values ( Fig. 70-11 ) up to PaO2 values of 1700 mm Hg. The following equations may be used to predict PaO2 at pressure.
The alveolar gas equation is required to calculate PAO2
:
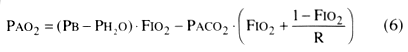
where PB and PH2
O
are, respectively, the ambient and saturated water vapor pressure and R is the respiratory
exchange ratio. If FIO2
= 0.2, R = 0.8,
and body temperature = 37°C, Equation 6 can be simplified to
PAO2
= (PB
− 47) · 0.2 − 1.2 · PCO2
(7)
Having calculated PAO2
and measured PaO2
, the a/A
ratio can then be obtained at 1 ATA. The predicted PaO2
at increased ambient pressure while breathing 100% O2
may then be derived
from Equation 7:
PaO2 (pred)
=
a/A · [(760 · ATA − 47) −
PaCO2
] (8)
where ATA is the chamber pressure in atmospheres absolute. Although dose-response
curves for HBO are not yet available, a reasonable aim is to achieve a PaO2
of 1000 mm Hg or higher for routine chronic therapy and as high a level as possible
for the treatment of acute necrotizing infections.
A better monitor of tissue oxygenation may be mixed venous PO2 (Pv̄O2 ), which in the absence of shunts, may be a reasonably accurate estimate of mean tissue PO2 . [174]
 Figure 70-11
Measured versus predicted PaO2
at increased ambient pressure. Predicted PaO2
is calculated from room-air arterial blood gases, assuming that the arterial-alveolar
PO2
ratio (PaO2
/PAO2
,
or a/A ratio) is a constant. Data are shown both
for persons with normal lungs (a/A ratio ≥0.75)
and for patients with gas exchange abnormalities (a/A
ratio <0.75). It is evident that PaO2
predicted in this way is close to the actual measured PaO2
.
(From Moon RE, Camporesi EM, Shelton DL: Prediction of arterial PO2
during hyperbaric treatment. In Bove AA, Bachrach
AJ, Greenbaum LJ Jr [eds]: Underwater and Hyperbaric Physiology IX. Proceedings
of the Ninth International Symposium on Underwater and Hyperbaric Physiology. Bethesda,
MD, Undersea and Hyperbaric Medical Society, 1987, p 1127.)
Figure 70-11
Measured versus predicted PaO2
at increased ambient pressure. Predicted PaO2
is calculated from room-air arterial blood gases, assuming that the arterial-alveolar
PO2
ratio (PaO2
/PAO2
,
or a/A ratio) is a constant. Data are shown both
for persons with normal lungs (a/A ratio ≥0.75)
and for patients with gas exchange abnormalities (a/A
ratio <0.75). It is evident that PaO2
predicted in this way is close to the actual measured PaO2
.
(From Moon RE, Camporesi EM, Shelton DL: Prediction of arterial PO2
during hyperbaric treatment. In Bove AA, Bachrach
AJ, Greenbaum LJ Jr [eds]: Underwater and Hyperbaric Physiology IX. Proceedings
of the Ninth International Symposium on Underwater and Hyperbaric Physiology. Bethesda,
MD, Undersea and Hyperbaric Medical Society, 1987, p 1127.)
Normal values for pH and PCO2
under hyperbaric conditions are the same as they are at 1 ATA. This relationship
has been shown to be valid up to an ambient pressure of 66 ATA.[175]
PCO2
(and hence pH) should not be expected
to change significantly in blood samples that are decompressed, as noted earlier.
Therefore, values of
|
|
Ambient Pressure (ATA) | FIO2 | PaO2 (mm Hg) | Pv̄O2 (mm Hg) | Sv̄O2 (%) | Cardiac Output * (L/min) | Heart Rate (Beats/min) |
|---|---|---|---|---|---|---|---|
| A | 1 | 2 L/min | 130 | 33 | 61 | 3.7 | 102 |
| B | 2.5 | 1.0 | >1000 | 45 | 79 | 2.6 | 95 |
| C | 2.5 | 1.0 | >1000 | 56 | 88 | 3.6 | 101 |
Mechanical ventilation in a hyperbaric environment presents a variety of challenges. The ideal requirements for ventilation include small size, no electrical requirement, no use of flammable lubricants, ability to operate on a volume-cycled basis over a wide range of tidal volumes and respiratory rates, minimal modification needed for installation, and ability to provide positive end-expiratory pressure, as well as ventilate in intermittent mandatory ventilation and assist/control modes.[176] Additionally, the ideal gas source to actuate the ventilator should minimize the risk of combustion caused by buildup of static electricity.
As ambient pressure increases, gas density is proportionately
raised whereas gas viscosity changes relatively little. Therefore, in regions of
turbulent flow (i.e., in the large airways), airway resistance would be expected
to increase. Measurements of respiratory conductance (the reciprocal of resistance)
during tidal breathing[177]
indicate that it varies
with gas density according to the following formula:
G = G0
ρκ
(9)
where G is lung conductance at gas density ρ, G0
is conductance at
gas density 1.1 g/L (1 ATA), and κ is a constant that has been found to have
a mean value of -0.39. At 6 ATA this equation predicts that lung conductance would
decrease by 50%, which is equivalent to a doubling of pulmonary resistance. The
increased airway resistance leads to a decrement in maximum voluntary ventilation.
[175]
[178]
In
addition,
the higher gas density results in less efficient distribution of ventilation manifested
as an increase in physiologic dead space.[175]
[179]
The effects of these two phenomena include higher airway pressure during mechanical
ventilation and an increased ventilatory requirement. If ventilator settings are
not adjusted to compensate for the higher dead space, a rise in arterial PCO2
will occur.
Several types of ventilators have been used and tested in hyperbaric chambers. Pressure-cycled devices such as the Bird ventilator have been used with some success because their compactness admirably fulfills the requirement for small size. However, continual adjustment of rate and cycling pressure is necessary with changes in ambient pressure. Systematic evaluation of two Bird ventilator models and a Mark 2 model modified for hyperbaric use
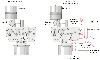 Figure 70-12
Monaghan 225 ventilator modified for use in a hyperbaric
chamber. A gas-mixing system with two ball rotameters has been added to provide
the inspired gas, which enters the ventilator through a conduit attached to the air
intake. The FIO2
setting has been permanently
set at 21% to force the ventilator to use the externally mixed gas as the breathing
gas. The FIO2
adjustment knob has been
removed. A 5-L reservoir bag is attached to the patient gas circuit. A reservoir
relief valve prevents overpressurization of the reservoir bag. A subambient pressure
relief valve allows entrainment of room air if insufficient gas is provided to the
reservoir bag. (From Moon RE, Bergquist LV, Conklin B, Miller JN: Monaghan
225 ventilator use under hyperbaric conditions. Chest 89:846, 1986.)
Figure 70-12
Monaghan 225 ventilator modified for use in a hyperbaric
chamber. A gas-mixing system with two ball rotameters has been added to provide
the inspired gas, which enters the ventilator through a conduit attached to the air
intake. The FIO2
setting has been permanently
set at 21% to force the ventilator to use the externally mixed gas as the breathing
gas. The FIO2
adjustment knob has been
removed. A 5-L reservoir bag is attached to the patient gas circuit. A reservoir
relief valve prevents overpressurization of the reservoir bag. A subambient pressure
relief valve allows entrainment of room air if insufficient gas is provided to the
reservoir bag. (From Moon RE, Bergquist LV, Conklin B, Miller JN: Monaghan
225 ventilator use under hyperbaric conditions. Chest 89:846, 1986.)
Two safety considerations are worthy of mention. First, in any ventilator delivering enriched O2 , there is a potential for O2 buildup within the housing. A fire hazard may exist if electrical components surrounded by a high O2 concentration overheat or spark. Moreover, leakage of O2 from the housing into the chamber may cause rapid O2 buildup in the chamber atmosphere. The effect of this leakage on the chamber's O2 concentration can be minimized in practice by inserting scavenging tubing into the ventilator cowling to remove a large proportion of the leaking O2 through the chamber overboard dump system. Modification of the Monaghan ventilator to compressed air actuation [176] eliminates O2 leakage other than from the patient's expired gas. The other safety consideration is the reliability and safety of electrical components. Large sealed
Air-filled endotracheal tube cuffs tend to lose volume during compression and re-expand during decompression. Therefore, either continuous adjustment of the air pressure within the cuff manually or the easier measure of filling the cuff with water will obviate these difficulties.
|
|
|
|
|
|
|
|
|
|
|
|
|