|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The expired minimum alveolar concentration (MAC) of inhaled anesthetics required in pediatric patients changes with age ( Fig. 60-6 ).[45] [46] [47] [48] [49] [50] [51] [52] [53] [54] Carefully controlled studies show that the anesthetic requirement is lower for premature than for term neonates and lower for term neonates than for a 3-month-old. Infants have a higher MAC than older children or adults do; the reasons have not been adequately explained. This fact, combined with the need for deeper planes of anesthesia to achieve satisfactory conditions for endotracheal intubation, places the infant in a precarious position in that the margin between anesthetic overdose (from a cardiovascular standpoint) and inadequate depth of anesthesia (for endotracheal intubation) is small. [55] Avoidance of controlled respirations until an intravenous line is inserted, rapid reduction in the delivery of inspired anesthetic drug, especially with the initiation of controlled respirations after the administration of a muscle relaxant, and in some cases, substitution of narcotics for an inhaled drug are practices that improve safety.[56]
The uptake of potent anesthetics is more rapid in children because of increased respiratory rates and cardiac index and a greater proportional distribution of cardiac output to vessel-rich organs. This rapid rise in blood anesthetic levels combined with the functional immaturity of cardiac development probably explains in part why it is so easy to give an overdose to infants and toddlers. Age-related differences in blood-gas partition coefficients may also facilitate a more rapid rise in alveolar concentration in infants.[57] [58] Other factors include the state of hydration (e.g., excessive fasting would make a small infant relatively dehydrated) and the type of anesthesia circuit used. For example, a Mapleson D has a smaller volume than a circle system and therefore less volume to achieve
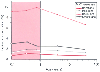 Figure 60-6
The minimum alveolar concentration (MAC) for four commonly
used inhaled anesthetics is plotted versus age. Note that MAC is highest in infants
3 to 6 months of age, the reasons for which are not clear. (Data extracted
from a number of studies.[45]
[47]
[49]
[50]
[51]
[52]
[53]
[54]
)
Figure 60-6
The minimum alveolar concentration (MAC) for four commonly
used inhaled anesthetics is plotted versus age. Note that MAC is highest in infants
3 to 6 months of age, the reasons for which are not clear. (Data extracted
from a number of studies.[45]
[47]
[49]
[50]
[51]
[52]
[53]
[54]
)
| Agent | Maximum Vaporizer Output (%) | MAC (%) | Maximum Possible MAC Multiples |
|---|---|---|---|
| Halothane | 5 | 0.87 | 5.75 |
| Isoflurane | 5 | 1.20 | 4.2 |
| Sevoflurane | 8 | 3.3 | 2.42 |
| Desflurane | 18 | 9.16 | 1.96 |
| From Coté CJ, Lugo RA, Ward RM: Pharmacokinetics and pharmacology of drugs in children. In Coté CJ, Todres ID, Goudsouzian NG, et al (eds): A Practice of Anesthesia for Infants and Children, 3rd ed. Philadelphia, WB Saunders, 2001, pp 121–171. | |||
Sevoflurane has a gas partition coefficient similar to that of nitrous oxide. Broad clinical experience has shown that it is less pungent than isoflurane and desflurane, and some authors believe that it is superior or equivalent to halothane for gaseous induction.[59] [60] [61] [62] [63] As with all potent volatile anesthetics, the MAC is highest in infants: 3.3% for neonates, 3.2% for infants 1 to 6 months old, and 2.5% for children older than 6 months.[45] [47] Sevoflurane and halothane are approximately equivalent in terms of airway complications during induction of anesthesia, but the rate of induction is slightly more rapid with sevoflurane. When data are pooled from a variety of studies, there is no difference in the incidence of laryngospasm or bronchospasm, but the incidence of coughing during induction with sevoflurane is lower ( Table 60-3 ). Sevoflurane and halothane produce dose-related respiratory depression; however, halothane produces a decrease in tidal volume and an increase in respiratory rate, whereas sevoflurane decreases both respiratory rate and tidal volume.[78] [79] I have often seen the need to assist respirations during the early induction stage in sevoflurane-anesthetized patients. Unless the inspired concentration is markedly reduced, such assistance then increases the potential for anesthetic overdose while attempts are made to establish intravenous access. [80]
Sevoflurane and halothane also have a different cardiovascular profile. Children older than 3 years have an increase in heart rate and no change in systolic blood pressure with sevoflurane, whereas with halothane, the heart rate does not change but systolic blood pressure decreases.[64] [65] [81] It should be noted that the very groups
|
|
Sevoflurane | Halothane |
|
||||
|---|---|---|---|---|---|---|---|
| Problem | Yes | No | % | Yes | No | % | Chi Square |
| Laryngospasm | 22 | 773 | 2.8 | 22 | 601 | 3.5 | .503 |
| Breath-holding | 33 | 635 | 4.9 | 34 | 439 | 7.2 | .143 |
| Coughing | 42 | 662 | 6.0 | 52 | 454 | 10.3 | .008 |
| Excitement during induction | 92 | 556 | 14.2 | 58 | 423 | 12.0 | .338 |
| Bronchospasm | 2 | 604 | 0.33 | 2 | 436 | 0.46 | .856 |
| Excitement during emergence | 169 | 645 | 20.8 | 102 | 573 | 15.1 | .006 |
| Data from references [59] [60] [62] [64] [65] [66] [67] [68] [69] [70] [71] [72] [73] [74] [75] [76] [77] . | |||||||
The safety of all potent anesthetics relates to how the drugs are used, the experience of the person administering the anesthesia, and other less obvious factors. I generally induce anesthesia with sevoflurane, and if the infant becomes apneic, I rapidly reduce the inspired concentration of sevoflurane while gently assisting respirations. After intravenous access is established, I administer a muscle relaxant and then, after endotracheal intubation, change to halothane or isoflurane for maintenance to reduce costs. Because multiple studies have found no difference in time to discharge (street readiness), there seems little logic in using sevoflurane for the entire procedure except for very brief anesthesia.[60] [66] [85] [86]
Several other issues related to sevoflurane are worthy of mention. Metabolic breakdown and release of fluoride do not appear to be significant issues even with prolonged anesthesia.[45] [65] However, the production of toxic metabolites as a result of interaction with the carbon dioxide absorbent must be considered.[87] [88] Compound A appears to be nephrotoxic in animal models[89] ; however, conflicting data have been presented regarding the clinical importance of this observation.[88] [90] [91] [92] [93] [94] [95] [96] Studies of low-flow (2 L/min) and prolonged anesthesia have not demonstrated significant alterations in the usual markers of renal function. It appears that sevoflurane is a safe anesthetic even for prolonged surgical procedures. Fresh gas flows of less than 1 L/min are not recommended.[97] The use of new carbon dioxide absorbents will probably eliminate this concern.[98] [99] [100] [101]
Another concern is the apparently higher incidence of emergence agitation than noted with halothane (see Table 60-3 ).[60] [66] [67] [68] [69] [70] [71] [72] [73] [102] [103] Unfortunately, because of widespread confusion regarding definitions and descriptions of agitation/delirium, it is difficult to compare studies. Such a response during emergence from sevoflurane anesthesia is not related to pain, appears to be inversely related to age, and is especially frequent in children 5 years or younger.[67] [69] [103] [104] Some investigators have reported a lower incidence with midazolam premedication and the administration of clonidine (oral or epidural), ketorolac, or fentanyl.[68] [70] [105] [106] Another issue of interest is reports of seizure-like activity during induction with sevoflurane.[107] [108] At least one carefully conducted study has not documented seizure-like electroencephalographic activity, thus suggesting that these abnormal movements are not central in origin. [109]
One personal observation is that airway reflexes are inadequately suppressed for bronchoscopy performed with spontaneous ventilation; supplementation with intravenous propofol or muscle relaxation (or both) seems to be required with sevoflurane. Halothane appears to still be a superior inhaled drug for this procedure because higher MAC multiples may be used to maintain the concentration of inhaled drug even in the face of large losses when the facemask is removed to allow insertion of the bronchoscope. The longer elimination half-life with halothane also contributes to its advantage for procedures in which the facemask will be intermittently applied, such as suture removal from a cleft lip repair.
Halothane does not have a noxious smell and is still commonly used for the gaseous induction of anesthesia. However, sevoflurane appears to be slightly less noxious and is increasingly being used for induction, with a change to halothane or isoflurane after induction because of cost restraints. Studies have found no clinically important differences among halothane, enflurane, and isoflurane in the rapidity of awakening.[61] [65] [86] [102] [110] A statistically significant, but clinically unimportant difference is nearly always found in the rapidity of awakening when comparing halothane with either desflurane or sevoflurane (usually 3 to 5 minutes).[61] [65] [86] [102] [110] I often induce anesthesia with sevoflurane and then use halothane for maintenance to reduce costs. Most importantly, airway-related problems occur less frequently with halothane and sevoflurane than with enflurane, isoflurane, or desflurane.[110] [111] [112] [113] Halothane and sevoflurane are the anesthetics of choice for the gaseous induction of anesthesia.
A 1987 report describing seven cases (one fatal) of "halothane hepatitis" concluded that children should not undergo repeated exposure to halothane (also see Chapter 8 ).[114] This report must be placed in proper perspective. To date, millions of children have been anesthetized with halothane. Perhaps a dozen instances of
Another concern with halothane is sensitization of the myocardium to arrhythmias because of exogenous and endogenous catecholamines. Most arrhythmias associated with halothane anesthesia in children are caused by either hypercapnia or an inadequate level of anesthesia.[119] Up to 10 µg/kg of epinephrine may be used with minimal risk of cardiac arrhythmias in pediatric patients.[120] In fact, I like the arrhythmogenic effects of halothane because the development of an arrhythmia suggests that the patient is inadequately anesthetized or is hypercapnic. In addition, the heart rate is generally stable or slightly decreased. If tachycardia develops in a halothane-anesthetized patient, inadequate anesthesia or hypovolemia is usually indicated. This situation is distinctly different from that in isoflurane, desflurane, or sevoflurane, which can all directly cause tachycardia.
Halothane is a potent myocardial depressant that can have profound effects on neonates and children with congenital heart disease.[56] [121] Such depression is responsible for the occasional inability to give critically ill patients sufficient concentrations of anesthetics to provide "anesthesia" without inducing severe hypotension. In this circumstance, the liberal use of short-acting narcotics with light concentrations of halothane generally provides the desired response. The POCA study reported a greater number of anesthetic-induced cardiac arrests with halothane; however, several cases also occurred with sevoflurane.[80] The use of controlled ventilation (probably without reducing the inspired concentration of anesthetic drug) was a common observation. Because sevoflurane was recently introduced in the United States at the time of the study, it is difficult to interpret the importance of the association of these cardiac events and the use of halothane. It is of interest that both halothane and sevoflurane have been shown to depress cardiac function, but sevoflurane is considered to be less of a myocardial depressant.[82] [83] However, at both 1 and 1.5 MAC in children, no significant difference was found in mean arterial blood pressure[83] ; in infants, no difference was found at 1 MAC, but there was a difference at 1.5 MAC. It should be further noted that simple administration of atropine will abolish these differences.
Isoflurane is claimed to have some advantages over halothane: less myocardial depression, preservation of the heart rate, and a greater reduction in the cerebral metabolic rate for oxygen.[122] [123] [124] These properties may be beneficial in selected patients. The major disadvantage of isoflurane is its noxious smell, which is unacceptable to many pediatric patients, and the greater incidence of airway-related events (laryngospasm, coughing).[112] [113] Hypertension is also occasionally observed, especially in teenagers, when the inspired concentration is rapidly increased or when there is a sudden change from sevoflurane to isoflurane. The probable mechanism is similar to that of desflurane: stimulation of pulmonary irritant receptors causing increased sympathetic activity and stimulation of the renin-angiotensin system.[125] In these patients I have also occasionally observed a diffuse rash primarily on the torso. All signs and symptoms regress with a reduction in the inspired concentration of isoflurane.
Desflurane has a gas partition coefficient similar to that of nitrous oxide.[51] Unfortunately, it has been found to cause an unacceptable incidence of laryngospasm (≅50%) during the gaseous induction of anesthesia in children.[111] Gaseous induction of anesthesia with halothane or sevoflurane and then changing to desflurane for maintenance and wake-up may be reasonable. This changeover, unlike a similar change to halothane or isoflurane, may be clinically important because the gas partition coefficient clearly favors rapid excretion. However, it would appear to be more important to change to desflurane for longer procedures, in which there is the potential for the accumulation of potent drug within fat, than for brief procedures, in which such accumulation is less likely. The more rapid awakening may also be advantageous for neurosurgical and spinal fusion procedures, for which early assessment of mental and neurologic status is important. One study of pediatric adenoidectomy patients demonstrated a more rapid wake-up with desflurane than with sevoflurane or halothane, but desflurane was also associated with the highest rate of emergence agitation. [102] Nasal fentanyl (2 µg/kg) reduces the incidence of emergence agitation, but my impression is that it also increases the incidence of postoperative vomiting.[105]
The MAC for desflurane is age dependent: 9.2% for neonates, 9.4% for infants 1 to 6 months old, 9.9% for infants 6 to 12 months of age, 8.7% for 1- to 3-year-olds, and 8% for 5- to 12-year-olds.[51] Interestingly, nitrous oxide does not appear to contribute to the MAC of desflurane to the same degree that it does with other potent volatile anesthetics.[46] Nitrous oxide undergoes virtually no hepatic metabolism, which clearly sets it apart from the other currently available potent anesthetics. Concern for the potential for carbon monoxide poisoning because of the dry carbon dioxide absorbent (also possible with isoflurane) can be prevented by rehydration of barium hydroxide lime (Baralyme) before use with desflurane.[126] [127]
|
|
|
|
|
|
|
|
|
|
|
|
|