|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This section will focus on the impact of volatile and intravenous anesthetics on liver blood flow and hepatic function. Halothane hepatitis and related volatile anesthetic-induced hepatotoxicity are discussed in Chapter 8 .
Volatile anesthetics variably affect blood flow to the liver, whereas intravenous anesthetics and opioids have a less significant impact, although their propensity to alter hepatic blood flow has been less extensively investigated than that of volatile agents. Interpretation of clinical and experimental results is further limited by numerous potential confounding variables that may influence hepatic blood flow and liver function, including the animal species studied, subject age, intravascular volume status, type of mechanical ventilation used, body position at the time of physiologic measurements, simultaneous surgical procedures, changes in blood pressure, concomitant use of vasopressors and local anesthetics, and alterations in hemoglobin and arterial oxygen concentrations. Despite these variables, reasonably firm conclusions can be reached regarding the impact and probable clinical significance of anesthetic agents on hepatic function in normal and cirrhotic patients.
The impact of volatile anesthetics on hepatic blood flow (including hepatic arterial and portal venous blood flow), oxygen delivery, and hepatic oxygen supply-demand ratios has been extensively evaluated for all the major volatile anesthetics, primarily in rat and pig experimental models. Available data from human studies also support the findings of these early experimental investigations.[2] [3] A variety of measurement techniques have been used to assess hepatic and portal venous blood flow, but plasma clearance of indocyanine green is most commonly used to assess hepatic blood flow. Transesophageal echocardiography is a new investigative tool that has been used to evaluate hepatic vein flow, but it can be considered only an indirect measurement of hepatic perfusion and oxygenation. A novel technique involving pulsed Doppler probes implanted in animals and in humans undergoing cholecystectomy has allowed accurate measurement of hepatic arterial and portal vein blood flow.[4] Most anesthetics decrease portal blood flow (PBF) because of decreased cardiac output; however, hepatic arterial blood flow (HABF) may increase, though often not sufficiently to restore total hepatic blood flow (THBF) to normal values.[5] THBF is equal to the sum of PBF and HABF. A consistent finding from most studies has been a reduction in mean arterial pressure (MAP) and a modest decrease in cardiac output with all volatile anesthetics, but a more pronounced reduction in THBF, PBF, and HABF with halothane than with enflurane, isoflurane, and sevoflurane ( Fig. 55-1 ).[2] [3] [4] [6] [7] [8] [9] [10] These changes generally apply across a range of minimum alveolar concentrations (MACs). Volatile anesthetics also alter portal venous and hepatic arterial vascular resistance to variable degrees, which in conjunction with decreases in cardiac output, MAP, and mesenteric sympathetic tone, modifies hepatic vascular supply.[11]
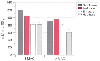 Figure 55-1
Impact of halothane, enflurane, isoflurane, and sevoflurane
at 1.0 and 2.0 minimum alveolar concentration (MAC) on total hepatic blood flow (THBF)
in dogs. THBF decreased with increasing anesthetic concentrations of each volatile
agent. Sevoflurane and isoflurane were similar in their modest effect on THBF, whereas
halothane caused a dramatic decrease in THBF, especially at 2-MAC exposure. *Differs
from sevoflurane and isoflurane at comparable MAC values (P
< .05). (Data from Frink EJ, Morgan SE, Coetzee A, et al: The effects
of sevoflurane, halothane, enflurane, and isoflurane on hepatic blood flow and oxygenation
in chronically instrumented greyhound dogs. Anesthesiology 76:85–90, 1992.)
Figure 55-1
Impact of halothane, enflurane, isoflurane, and sevoflurane
at 1.0 and 2.0 minimum alveolar concentration (MAC) on total hepatic blood flow (THBF)
in dogs. THBF decreased with increasing anesthetic concentrations of each volatile
agent. Sevoflurane and isoflurane were similar in their modest effect on THBF, whereas
halothane caused a dramatic decrease in THBF, especially at 2-MAC exposure. *Differs
from sevoflurane and isoflurane at comparable MAC values (P
< .05). (Data from Frink EJ, Morgan SE, Coetzee A, et al: The effects
of sevoflurane, halothane, enflurane, and isoflurane on hepatic blood flow and oxygenation
in chronically instrumented greyhound dogs. Anesthesiology 76:85–90, 1992.)
Although both halothane and isoflurane decrease MAP and PBF, halothane has a more consistently dramatic impact on HABF. Halothane appears to cause vasoconstriction in the hepatic arterial vascular bed, as reflected by an increase in hepatic arterial resistance.[4] [12] In vivo videomi-croscopy after halothane exposure in rats has demonstrated reduced sinusoidal blood flow as a result of decreased sinusoidal diameter, thus providing direct evidence of vasoconstriction at the microvascular level.[12] In contrast, isoflurane increased flow velocity in hepatic sinusoids and in this way preserved microvascular blood flow more than halothane or enflurane did.[12] Interestingly, Benumof and colleagues[13] demonstrated markedly reduced HABF after halothane administration in two patients during hepatic arteriography; hepatic blood flow returned to normal 20 minutes after discontinuation of halothane anesthesia.
Halothane also reduces hepatic oxygen delivery and hepatic venous oxygen saturation ( Fig. 55-2 ). [14] These changes are related to decreased MAP and more dramatic reductions in cardiac output with halothane than with any other volatile anesthetics.[5] Gelman and associates [9] demonstrated that during surgical stress in pigs, fentanyl or isoflurane anesthesia, which decreases blood pressure by less than 30%, provides adequate oxygen supply whereas halothane anesthesia, which produces decreases in blood pressure greater than 30%, provides inadequate hepatic oxygen supply. This finding also applied to episodes of hepatic ischemia, where fentanyl or isoflurane provided more protection than halothane or enflurane did. In addition to vascular changes, hepatic function measured by serum transaminase levels also suggests an unfavorable impact of halothane versus isoflurane.[3]
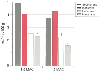 Figure 55-2
Impact of halothane, enflurane, isoflurane, and sevoflurane
at 1.5 and 2.0 minimum alveolar concentration (MAC) on hepatic arterial oxygen delivery
in chronically instrumented dogs. Halothane produced the greatest reduction in hepatic
arterial oxygen delivery, whereas sevoflurane and isoflurane had insignificant effects
on oxygen delivery at any MAC level. *Differs from isoflurane and sevoflurane
at comparable MAC values (P < .05). †Differs
from sevoflurane at comparable MAC values (P <
.05). (Data from Frink EJ, Morgan SE, Coetzee A, et al: The effects of
sevoflurane, halothane, enflurane, and isoflurane on hepatic blood flow and oxygenation
in chronically instrumented greyhound dogs. Anesthesiology 76:85–90, 1992.)
Figure 55-2
Impact of halothane, enflurane, isoflurane, and sevoflurane
at 1.5 and 2.0 minimum alveolar concentration (MAC) on hepatic arterial oxygen delivery
in chronically instrumented dogs. Halothane produced the greatest reduction in hepatic
arterial oxygen delivery, whereas sevoflurane and isoflurane had insignificant effects
on oxygen delivery at any MAC level. *Differs from isoflurane and sevoflurane
at comparable MAC values (P < .05). †Differs
from sevoflurane at comparable MAC values (P <
.05). (Data from Frink EJ, Morgan SE, Coetzee A, et al: The effects of
sevoflurane, halothane, enflurane, and isoflurane on hepatic blood flow and oxygenation
in chronically instrumented greyhound dogs. Anesthesiology 76:85–90, 1992.)
Volatile anesthetic-induced alterations in hepatic blood flow are, in part, mediated by an autoregulatory mechanism that maintains constant THBF. This physiologic adaptation is termed the hepatic arterial buffer response (HABR), which matches reductions in PBF with increases in HABF to maintain total blood flow to the liver constant in the face of profound hypovolemia, indirect effects of major abdominal surgery, or severe hemorrhage.[15] Halothane appears to disturb this compensatory response, whereas sevoflurane and isoflurane maintain HABR.[15] [16] Sevoflurane further suppresses the increase in hepatic arterial vascular resistance better than halothane does and thus maintains HABF more effectively.[17] Sevoflurane is also consistently equivalent or superior to isoflurane in maintaining HABF, hepatic O2 delivery, and O2 delivery-to-consumption ratios. [10] [18] [19] In addition, laboratory studies demonstrate insignificant changes in conventional liver function test results after exposure to isoflurane or desflurane.[3] [20] [21]
The encouraging results obtained with sevoflurane versus older volatile anesthetics are further supported by the observation that compound A produced by prolonged, low-flow sevoflurane anesthesia does not adversely affect hepatic function in adult surgical patients as determined by liver function testing or measurement of arterial ketone body ratios, a putative measure of hepatocyte function.[22] [23] [24] [25] [26] In the largest clinical study of sevoflurane, Bito and coauthors[26] noted mild postoperative increases in bilirubin and transaminase values in 100 surgical patients receiving low- and high-flow sevoflurane or isoflurane anesthesia, but no evidence of clinical hepatotoxicity. It is notable that routine liver function test results are often mildly elevated after surgery and anesthesia using a variety of different volatile anesthetics, although the specific contribution of the anesthetic itself is debatable. [3] [22] [23] [27]
Administration of either sevoflurane[24] [25] [28] or desflurane [29] [30] to human volunteers not undergoing surgery produced no significant abnormalities in liver function test results, thus suggesting that other perioperative surgical factors may induce mild, transient alterations in plasma transaminase levels. In fact, early human investigations found steep declines in estimated hepatic blood flow immediately after the induction of anesthesia that correlated with the decrease in blood pressure[31] ; hepatic blood flow rapidly returned to normal soon after the start of surgery, thus suggesting global reductions in cardiac output and blood pressure as mechanisms responsible for the reduced hepatic blood flow rather than a sustained adverse influence of specific volatile or intravenous anesthetics on hepatic blood flow.
Desflurane has effects similar to those of isoflurane on hepatic blood flow and function when assessed in animal and human investigations. In chronically instrumented dogs, Merin and associates[32] demonstrated slight, but significant decreases in THBF at 1.75 MAC as a result of decreased PBF, but no significant differences between desflurane and isoflurane in any measure of hepatic blood flow over a range of MAC values. Armbruster and coworkers[21] observed decreased THBF at 1 MAC desflurane in pigs, but only at levels of hypotension unlikely to be encountered clinically and without alterations in liver function test results. Other canine data suggest better preservation of THBF with desflurane than with halothane or
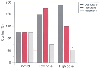 Figure 55-3
Influence of low- and high-dose desflurane, halothane,
and isoflurane on total hepatic blood flow (THBF) as measured with radioactive microspheres
in chronically instrumented dogs. In contrast to desflurane and isoflurane, which
increased THBF, halothane caused a significant decrease in THBF at high doses. *Significantly
different from conscious control (P < 0.05).
(Data from Hartman JC, Pagel PS, Proctor LT, et al: Influence of desflurane,
isoflurane and halothane on regional tissue perfusion in dogs. Can J Anaesth 39:877–887,
1992.)
Figure 55-3
Influence of low- and high-dose desflurane, halothane,
and isoflurane on total hepatic blood flow (THBF) as measured with radioactive microspheres
in chronically instrumented dogs. In contrast to desflurane and isoflurane, which
increased THBF, halothane caused a significant decrease in THBF at high doses. *Significantly
different from conscious control (P < 0.05).
(Data from Hartman JC, Pagel PS, Proctor LT, et al: Influence of desflurane,
isoflurane and halothane on regional tissue perfusion in dogs. Can J Anaesth 39:877–887,
1992.)
In contrast to healthy volunteers and surgical patients, substantially less information is available that describes the impact of anesthetics on hepatic function in patients with advanced liver disease. Limited data suggest that desflurane and isoflurane do not change perioperative liver function test results in adult surgical patients with chronic liver disease.[34] Isoflurane seems to be more efficient at preserving hepatic blood flow in cirrhotic animals than ketamine or halothane is,[35] although other studies show no difference in hepatic function in cirrhotic rats exposed to fentanyl, halothane, enflurane, or isoflurane.[36] However, based on the less favorable effects of halothane on both hepatic blood flow and hepatic function, its use should be avoided in patients with advanced liver disease. Given the current availability of suitable alternative volatile anesthetics and the overall declining use of halothane, this issue may become primarily of historical interest. Moreover, as a consequence of the hepatotoxic potential of halothane, many authorities currently consider it unjustifiable to use halothane in healthy adults or any patient with significant hepatic dysfunction.
In summary, the influence of volatile anesthetics on hepatic blood flow and function is complex and related not only to features unique to the anesthetic itself but also to other patient-related variables such as the severity of underlying liver dysfunction, the presence of advanced age, and the impact of surgical stress and intra-abdominal surgical manipulation. However, sevoflurane, desflurane, and isoflurane have been consistently shown to better preserve hepatic blood flow and function than halothane or enflurane does. Large-scale prospective studies will be necessary to better define the impact of
|
|
|
|
|
|
|
|
|
|
|
|
|