|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The rate of occurrence of venous air embolism (VAE) varies according to the procedure, the intraoperative position, and the method of detection used. During posterior fossa procedures performed in the sitting position,
Common sources of critical VAE are the major cerebral venous sinuses, in particular, the transverse, the sigmoid, and the posterior half of the sagittal sinus, all of which may be noncollapsible because of their dural attachments. Air may also enter through emissary veins, particularly from the suboccipital musculature, the diploic space of the skull (which can be violated by both the
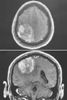 Figure 53-10
Horizontal (top) and
coronal magnetic resonance images of a parasagittal meningioma.[101]
Resection of meningiomas arising from the dural reflection overlying the sagittal
sinus or from the dura of the adjacent convexity or falx entails a risk of venous
air embolism because of the proximity of the sagittal sinus (the triangular structure
at the superior end of the interhemispheric fissure in the bottom
panel).
Figure 53-10
Horizontal (top) and
coronal magnetic resonance images of a parasagittal meningioma.[101]
Resection of meningiomas arising from the dural reflection overlying the sagittal
sinus or from the dura of the adjacent convexity or falx entails a risk of venous
air embolism because of the proximity of the sagittal sinus (the triangular structure
at the superior end of the interhemispheric fissure in the bottom
panel).
Monitors used for detection of VAE should provide (1) a high level of sensitivity, (2) good specificity, (3) a rapid response, (4) a quantitative measure of the VAE event, and (5) an indication of the course of recovery from the VAE event. The combination of precordial Doppler and expired CO2 monitoring meets these criteria and is the current standard of care. Doppler placement in a left or right parasternal location in the second to fourth intercostal space has a very high detection rate for gas embolization,[105] and when good heart tone is obtained, maneuvers to confirm adequate placement appear to be unnecessary. TEE is more sensitive to VAE than precordial Doppler is[106] ( Fig. 53-11 ) and offers the advantage of identifying right-to-left shunting of air.[100] [106] [107] [108] [109] However, its safety during prolonged use (especially with pronounced neck flexion) is not well established. Expired nitrogen analysis is theoretically attractive. However, the expired N2 concentrations involved in anything less than catastrophic VAE are very small and push the available instrumentation to the limits of its sensitivity.[110] Furthermore, effective application requires absolute freedom from air contamination of the ventilator and anesthetic circuit.
Figure 53-12 presents the physiologic and monitor response to an air embolic event. Table 53-6 offers an appropriate management response to such an event.
 Figure 53-11
Relative sensitivity of various monitoring techniques
to the occurrence of venous air embolism. BP, blood pressure; C.O., cardiac output;
CVP, central venous pressure; ECG, electrocardiogram; ET-CO2, end-tidal CO2
;
PAP, pulmonary artery pressure; T-ECHO, transesophageal echocardiography.
Figure 53-11
Relative sensitivity of various monitoring techniques
to the occurrence of venous air embolism. BP, blood pressure; C.O., cardiac output;
CVP, central venous pressure; ECG, electrocardiogram; ET-CO2, end-tidal CO2
;
PAP, pulmonary artery pressure; T-ECHO, transesophageal echocardiography.
 Figure 53-12
Responses of the electrocardiogram (ECG), arterial blood
pressure (BP), pulmonary artery pressure (PAP), pan-tidal CO2
concentration,
precordial Doppler, and central venous pressure (CVP) to the intravenous administration
of 10 cc of air over a 30-second period to an 11-kg dog.
Figure 53-12
Responses of the electrocardiogram (ECG), arterial blood
pressure (BP), pulmonary artery pressure (PAP), pan-tidal CO2
concentration,
precordial Doppler, and central venous pressure (CVP) to the intravenous administration
of 10 cc of air over a 30-second period to an 11-kg dog.
Essentially all patients who undergo sitting posterior fossa procedures
should have a right heart catheter. Although catastrophic, life-threatening VAE
is relatively uncommon, a catheter that permits immediate evacuation of an air-filled
heart will occasionally be the sine qua non for resuscitation. Latitudes are much
wider with the nonsitting positions, and it is frequently appropriate, after a documented
discussion with the surgeon, to omit the right heart catheter. The perceived risks
of VAE associated with the intended procedure and the patient's physiologic reserve
are the variables that will contribute to the decision. Microvascular decompression
of the fifth or
| 1. Prevent further air entry |
| Notify surgeon (flood or pack surgical field) |
| Jugular compression |
| Lower the head |
| 2. Treat the intravascular air |
| Aspirate via a right heart catheter |
| Discontinue N2 O |
| FIO2 : 1.0 |
| (Pressors/inotropes) |
| (Chest compression) |
Although some surgeons may ask that neck veins not be used, a skillfully placed jugular catheter is often acceptable. In a very limited number of patients, high ICP may make the head-down posture undesirable. In others, unfavorable anatomy with an increased likelihood of difficult cannulation and formation of hematomas may also encourage the use of alternative access sites. Access to the brachial veins has been greatly facilitated by the commercial availability of multiorifice catheters that use a Seldinger cannulation technique and a lengthy J-tipped guidewire to negotiate the axilla or deltopectoral groove.
The investigation of Bunegin and colleagues suggests that a multiorifice catheter should be located with the tip 2 cm below the superior vena cava (SVC)-atrial junction and a single-orifice catheter with the tip 3 cm above the SVC-atrial junction.[111] Although these small distinctions in location may be relevant for optimal recovery of small volumes of air when cardiac output is well maintained, for the recovery of massive volumes of air in the face of cardiovascular collapse, anywhere in the right atrium should suffice. Confirmation of right heart placement can be accomplished by (1) radiographs, (2) pull back from the right ventricle while monitoring intravascular pressure, or (3) intravascular electrocardiography (ECG).[112] Although no literature in support of the practice has been published, with catheter access through the right internal jugular vein, measured placement to the level of the second right intercostal space should suffice when the catheter passes readily. The intravascular ECG technique makes use of the fact that an ECG "electrode" placed in the middle of the right atrium will initially "see" an increasing positivity as the developing P-wave vector approaches it ( Fig. 53-13 ) and then an increasing negativity as the wave of atrial depolarization passes and moves away from it. The resultant biphasic P wave is characteristic of a mid-atrial "electrode" position. The technique requires that the central venous pressure (CVP) catheter become an exploring ECG electrode, which is accomplished by filling the catheter with an electrolyte solution (bicarbonate is best) and attaching an ECG lead (the leg lead if lead II is selected) to the hub of the CVP catheter. Commercial CVP kits with an ECG adapter are available. The ECG configurations that will be observed at various intravascular locations are shown in Figure 53-13 . To minimize the microshock hazard, a battery-operated ECG unit is preferable, and unnecessary electrical apparatus should be detached from the patient during placement of the catheter.
Much concern has been raised about the possibility of the passage of air across the interatrial septum through a patent foramen ovale (PFO) (known to be present in approximately 25% of adults).[113] The concern is that this phenomenon carries the risk of major cerebral or coronary morbidity, or both, although the morbidity that can realistically be attributed to a paradoxical air embolism (PAE) has not been precisely defined. Even though the precise pressure required to open a PFO is not known with certainty, it is thought that the gradient necessary may be as much as 5 mm Hg. In a clinical investigation, Mammoto and coworkers observed that PAE occurred only in the context of major air embolic events, thus suggesting that significant increases in pressure in the right side of the heart are an important predisposing factor for the occurrence of PAE. [109] Several clinical investigations have examined factors that influence the right atrial-to-left atrial pressure gradient. The use of positive end-expiratory pressure (PEEP) was shown to increase the incidence of a positive right atrial pressure (RAP)-pulmonary capillary wedge pressure (PCWP) gradient,[114] and generous fluid administration (e.g., 2800 mL per patient versus 1220 mL per control patient[115] ) was shown
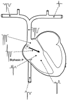 Figure 53-13
Electrocardiographic (ECG) configurations observed at
various locations when a central venous catheter is used as an intravascular ECG
electrode. The configurations in the figure will be observed when "lead II" is monitored
and the positive electrode (the leg electrode) is connected to the catheter. P indicates
the sinoatrial node. The heavy black arrow indicates
the P-wave vector. Note the equi-biphasic P wave when the catheter tip is in the
mid-right atrial position.[111]
Figure 53-13
Electrocardiographic (ECG) configurations observed at
various locations when a central venous catheter is used as an intravascular ECG
electrode. The configurations in the figure will be observed when "lead II" is monitored
and the positive electrode (the leg electrode) is connected to the catheter. P indicates
the sinoatrial node. The heavy black arrow indicates
the P-wave vector. Note the equi-biphasic P wave when the catheter tip is in the
mid-right atrial position.[111]
For those undertaking prepositioning detection of a PFO, it should be understood that TEE-based methods are more efficient in detecting a PFO than transthoracic echocardiographic techniques are. The greatest sensitivity is achieved with the combination of TEE contrast imaging and color Doppler imaging, in part because the former may fail to identify shunting if LAP is persistently higher than RAP at the time of study.[123]
It appears likely that air can occasionally traverse the pulmonary vascular bed to reach the systemic circulation.[124] [125] Transpulmonary passage is more likely to occur when large volumes of air are presented to the pulmonary vascular "filter."[126] Evidence also suggests that pulmonary vasodilators,[126] including volatile anesthetics, may lower the threshold for transpulmonary passage. [127] The magnitude of differences among anesthetics does not appear, to these reviewers, to mandate any related "tailoring" of anesthetic techniques. However, it does reinforce the notion that N2 O should be discontinued promptly after even apparently minor VAE events because of the possibility that air may reach the left-sided circulation through either a PFO or the pulmonary vascular bed.
As noted earlier, PEEP has been advocated in the past as a means of both reducing the incidence of VAE and responding to an acute VAE event to prevent further air entry. However, a study by Perkins and Bedford[114] presented data suggesting that PEEP increases the risk of PAE and that these data therefore argue against the use of PEEP in patients undergoing seated neurosurgical procedures. Furthermore, as the authors point out, even 10 cm H2 O of PEEP would be unlikely to result in positive venous pressure in cerebral venous structures, which may be as much as 25 cm above the heart. The ineffectiveness of PEEP[128] and the relative superiority of jugular venous compression[129] [130] in raising CVP have been confirmed by other investigations. An inflatable neck tourniquet available for rapid inflation in the event of VAE has been studied in animals and used in humans by Pfitzner and McLean. [131] The G-suit has also been reported to be more effective in producing increases in RAP than 10 cm H2 O of PEEP is and can do so without increasing the RAP-PCWP gradient.[132] This latter report is available only in a non-peer-reviewed (abstract) form. There are additional arguments against the acute use of PEEP in the event of VAE. The various investigations of the identification of PFOs have confirmed the efficacy of a Valsalva maneuver, in particular, its release, as a means of promoting paradoxical embolism.[117] [133] [134] In addition, the impairment in systemic venous return caused by the sudden application of substantial PEEP may be undesirable in the face of the cardiovascular dysfunction already caused by the VAE event.
It has been recommended that a patient who has sustained a hemodynamically significant VAE be placed in a lateral position with the right side up. The rationale is that air will remain in the right atrium where it will not contribute to air lock in the right ventricle and where it will remain amenable to recovery with a right atrial catheter. The first difficulty is that such repositioning is all but impossible with a patient in a pin head holder. In addition, the only systematic attempt to examine the efficacy of this maneuver, albeit performed in dogs, failed to identify any hemodynamic benefit.[135]
N2 O will diffuse into air bubbles trapped in the vascular tree, and accordingly, N2 O should be eliminated after a clinical VAE event to avoid aggravating the cardiovascular impact. As noted earlier, the PAE phenomenon adds an additional reason for eliminating N2 O after the occurrence of VAE. When a major VAE occurs, no matter how the RAP-LAP gradient was manipulated before the event, RAP will rise abruptly with respect to LAP,[136] and a major VAE will result in an acutely increased risk of PAE in patients with a PFO.[109] Should N2 O be used at all in patients at risk for VAE? Some will decide that it is the "path of least resistance" to simply avoid it and thereby avoid having to worry about the considerations that it creates. However, N2 O can be used with the knowledge that it neither increases the incidence of VAE[137] nor aggravates the hemodynamic response to VAE, provided that it is eliminated when VAE occurs. [138]
|
|
|
|
|
|
|
|
|
|
|
|
|