Mechanisms of Action
Volatile anesthetics relax airway smooth muscle by directly depressing
smooth muscle contractility. This action appears to result from direct effects on
bronchial epithelium and indirect inhibition of reflex neural pathways. The mechanisms
responsible for the direct depressant effects of volatile agents are unclear. Halothane
was initially believed to exert β-adrenoceptor agonist activity in airway smooth
muscle because halothane-induced reduction of airway resistance was blocked by sotalol.
[19]
However, these effects might have been specific
to the drug itself and not attributable to the drug class. Sotalol also might have
produced smooth muscle contraction because propranolol did not antagonize the relaxing
properties of halothane on acetylcholine-mediated bronchoconstriction.[20]
The concentration of ICa2+
is an important regulator of smooth muscle
reactivity. Several intracellular mediators responsible for Ca2+
mobilization
are potential sites for the action of volatile anesthetics. Halothane increases
cAMP concentrations, which may decrease intracellular free Ca2+
in airway
smooth muscle.[41]
This decrease in Ca2+
parallels the inhibitory effects of the volatile anesthetics on airway smooth muscle
tension.[39]
Effects of volatile anesthetics on proximal rather than distal
airways may be related to differential effects on voltage-dependent calcium channels
(VDCs) and the relative distribution of these channels. Long-lasting (L-type) VDCs
appear to be the predominant mechanism for Ca2+
entry in tracheal smooth
muscle, whereas transient (T-type) and L-type VDCs are present in bronchial smooth
muscle cells.[39]
[42]
Hypocapnia-induced contraction of tracheal and bronchial smooth muscle appears to
be mediated, at least in part, by VDCs.[43]
Yamakage
and coworkers[39]
demonstrated that isoflurane and
sevoflurane
inhibit both types of VDCs in a dose-dependent fashion, but their effects on T-type
VDCs in bronchial smooth muscle were much greater ( Fig.
6-2
). Early studies proposed that volatile anesthetics relax airway smooth
muscles by opening adenosine triphosphate (ATP)-sensitive potassium channels (KATP
).
However, halothane had minimal effects on tracheal smooth muscle KATP
channels.[44]
Other evidence suggests that the
differential effects of volatile anesthetics on tracheal compared with bronchial
smooth muscle may be related to various effects on Ca2+
-activated chloride
channel activity[45]
or differential sensitivities
of other K+
channel subtypes.[46]
In addition to effects on VDCs, halothane reduces ICa2+
by depleting sarcoplasmic reticulum Ca2+
.[47]
[48]
[49]
The direct
effects of halothane on IP3
concentrations remain controversial,[47]
[48]
but halothane-induced reductions in sarcoplasmic
reticulum Ca2+
appear to occur through IP3
and ryanodine receptor
channels.[48]
Halothane may reduce free Ca2+
in the cytoplasm or limit influx of Ca2+
across the cell membrane. Volatile
anesthetics also affect the function of G proteins in a variety of tissues. However,
halothane-induced depression of airway contractility occurs independent of pertussis
toxin-sensitive inhibitory G proteins that attenuate relaxation produced by β-adrenoceptor
agonists.[50]
Halothane decreased intracellular
Ca2+
concentration and Ca2+
influx during submaximal, but not
maximal, muscarinic stimulation.[51]
Warner and
coworkers[52]
suggested that halothane depletes
sarcoplasmic reticulum Ca2+
stores, but this effect
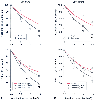 Figure 6-2
Effect of isoflurane (A)
and sevoflurane (B) on porcine tracheal versus bronchial
smooth muscle tension or inward Ca2+
current (ICa) through T-type and
L-type voltage-dependent Ca2+
channels (VDCs). There were no differences
in the inhibition of L-type VDCs. Both anesthetics had greater inhibitory effects
on T-type VDCs in bronchial smooth muscle. Symbols represent the mean ± SD.
*P < .05 versus 0 MAC. †P
< .05 versus tracheal smooth muscle in A and †P
< .05 versus L-type VDCs in B. (Adapted
from Yamakage M, Chen X, Tsuijiguchi N, et al: Different inhibitory effects of volatile
anesthetics on T- and L-type voltage-dependent Ca2+
channels in porcine
tracheal and bronchial smooth muscles. Anesthesiology 94:683, 2001.)
Figure 6-2
Effect of isoflurane (A)
and sevoflurane (B) on porcine tracheal versus bronchial
smooth muscle tension or inward Ca2+
current (ICa) through T-type and
L-type voltage-dependent Ca2+
channels (VDCs). There were no differences
in the inhibition of L-type VDCs. Both anesthetics had greater inhibitory effects
on T-type VDCs in bronchial smooth muscle. Symbols represent the mean ± SD.
*P < .05 versus 0 MAC. †P
< .05 versus tracheal smooth muscle in A and †P
< .05 versus L-type VDCs in B. (Adapted
from Yamakage M, Chen X, Tsuijiguchi N, et al: Different inhibitory effects of volatile
anesthetics on T- and L-type voltage-dependent Ca2+
channels in porcine
tracheal and bronchial smooth muscles. Anesthesiology 94:683, 2001.)
may not be the primary mechanism by which airway contractility is reduced ( Fig.
6-3
). Halothane also decreases Ca2+
sensitivity of smooth muscle
cell myocytes, resulting in a reduced contractile force at a constant Ca2+
concentration.[51]
Warner's group[53]
also demonstrated that halothane attenuates acetylcholine-induced Ca2+
sensitization in canine tracheal smooth muscle to a greater extent than does isoflurane
or sevoflurane. These findings are analogous to the differential effects of volatile
anesthetics on relaxing airway smooth muscle. Alterations in Ca2+
sensitivity
appear to be mediated by an increase in smooth muscle protein phosphatase[54]
and a modulation of G proteins, specifically Gq
and GI
, that
exert actions on cGMP.[55]
[56]
[57]
[58]
Halothane
suppresses the contraction of dog tracheal smooth muscle initiated by direct and
indirect electrical stimulation.[59]
The bronchoconstricting effects of low inhaled concentrations
of carbon dioxide were attenuated by inhaled, but not intravenous, halothane.[23]
These data suggest that volatile agents produce a direct action on the airway musculature
or local neural reflex arcs rather than centrally controlled reflex pathways. Halothane,
isoflurane, sevoflurane, and desflurane-induced dilatation of distal bronchial segments
partially depends on the presence of bronchial epithelium ( Fig.
6-4
).[28]
[60]
A prostanoid (e.g., prostaglandin E2
or I2
) or NO may mediate
the bronchodilatory effects of these volatile anesthetics. For example, isoflurane-mediated
bronchodilation appears to be more dependent on NO than prostanoids, but the
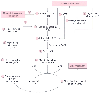 Figure 6-3
Proposed signaling pathways underlying volatile anesthetic
(specifically halothane)-induced bronchodilation or inhibition of muscarinic agonist-induced
contraction of airway smooth muscle, or both mechanisms. Signal transduction involving
Rho protein is supported by work from Warner and coworkers on a role of halothane
to decrease Ca2+
sensitivity rather than a change in ICa2+
content. CaM, calmodulin; ICa2+
intracellular Ca2+
; IP3, inositol
triphosphate; MLC, myosin light chain; MLCK, myosin light chain kinase; pMLC, phosphorylated
myosin light chain; Rho, monomeric G protein; ROK, Rho-associated kinase; RyR, ryanodine
channels; SMPP, smooth muscle protein phosphatase; SR, sarcoplasmic reticulum; VDC,
voltage-dependent Ca2+
channels; encircled plus sign,
excitatory action of muscarinic receptor agonist; encircled
up arrow, represents activation or increase due to volatile anesthetic;
encircled down arrow, inhibition or decrease due
to volatile anesthetic. (Adapted from Hanazaki M, Jones KA, Perkins WJ,
et al: Halothane increases smooth muscle protein phosphatase in airway smooth muscle.
Anesthesiology 94:129, 2001 and from Pabelick CM, Prakash YS, Kannan MS, et al:
Effects of halothane on sarcoplasmic reticulum calcium release channels in porcine
airway smooth muscle cells. Anesthesiology 95:207, 2001.)
Figure 6-3
Proposed signaling pathways underlying volatile anesthetic
(specifically halothane)-induced bronchodilation or inhibition of muscarinic agonist-induced
contraction of airway smooth muscle, or both mechanisms. Signal transduction involving
Rho protein is supported by work from Warner and coworkers on a role of halothane
to decrease Ca2+
sensitivity rather than a change in ICa2+
content. CaM, calmodulin; ICa2+
intracellular Ca2+
; IP3, inositol
triphosphate; MLC, myosin light chain; MLCK, myosin light chain kinase; pMLC, phosphorylated
myosin light chain; Rho, monomeric G protein; ROK, Rho-associated kinase; RyR, ryanodine
channels; SMPP, smooth muscle protein phosphatase; SR, sarcoplasmic reticulum; VDC,
voltage-dependent Ca2+
channels; encircled plus sign,
excitatory action of muscarinic receptor agonist; encircled
up arrow, represents activation or increase due to volatile anesthetic;
encircled down arrow, inhibition or decrease due
to volatile anesthetic. (Adapted from Hanazaki M, Jones KA, Perkins WJ,
et al: Halothane increases smooth muscle protein phosphatase in airway smooth muscle.
Anesthesiology 94:129, 2001 and from Pabelick CM, Prakash YS, Kannan MS, et al:
Effects of halothane on sarcoplasmic reticulum calcium release channels in porcine
airway smooth muscle cells. Anesthesiology 95:207, 2001.)
converse is true for halothane. Focal epithelial damage or inflammation may occur
in distal and small airways in patients with asthma or allergen exposure, and as
a consequence, the bronchodilatory response to volatile anesthetics may be reduced.
[6]
The greatest bronchodilatory action of volatile
agents in patients with chronic reactive airway disease occurs primarily in the proximal
rather than distal airways.
Stimulation of intrinsic airway nerves in vitro produces a cholinergic
contractile response that is inhibited by atropine. In addition to the direct effects
previously described, volatile anesthetic-induced bronchodilation occurs by modulation
of airway cholinergic neural transmission mediated through prejunctional and postjunctional
mechanisms.[59]
[61]
[62]
The combination of atropine and halothane
did
not increase airway caliber over that attained with either drug alone. These findings
suggest that halothane dilates airways by blocking vagal tone during unstimulated
conditions.[63]
A nonadrenergic and noncholinergic
bronchodilatory neural reflex believed to be mediated by NO[64]
was unaffected by halothane at concentrations of less than 1%.[65]
The direct antagonism or release of histamine[30]
also does not appear to play a role in volatile anesthetic-induced bronchodilation.
 Figure 6-2
Effect of isoflurane (A)
and sevoflurane (B) on porcine tracheal versus bronchial
smooth muscle tension or inward Ca2+
current (ICa) through T-type and
L-type voltage-dependent Ca2+
channels (VDCs). There were no differences
in the inhibition of L-type VDCs. Both anesthetics had greater inhibitory effects
on T-type VDCs in bronchial smooth muscle. Symbols represent the mean ± SD.
*P < .05 versus 0 MAC. †P
< .05 versus tracheal smooth muscle in A and †P
< .05 versus L-type VDCs in B. (Adapted
from Yamakage M, Chen X, Tsuijiguchi N, et al: Different inhibitory effects of volatile
anesthetics on T- and L-type voltage-dependent Ca2+
channels in porcine
tracheal and bronchial smooth muscles. Anesthesiology 94:683, 2001.)
Figure 6-2
Effect of isoflurane (A)
and sevoflurane (B) on porcine tracheal versus bronchial
smooth muscle tension or inward Ca2+
current (ICa) through T-type and
L-type voltage-dependent Ca2+
channels (VDCs). There were no differences
in the inhibition of L-type VDCs. Both anesthetics had greater inhibitory effects
on T-type VDCs in bronchial smooth muscle. Symbols represent the mean ± SD.
*P < .05 versus 0 MAC. †P
< .05 versus tracheal smooth muscle in A and †P
< .05 versus L-type VDCs in B. (Adapted
from Yamakage M, Chen X, Tsuijiguchi N, et al: Different inhibitory effects of volatile
anesthetics on T- and L-type voltage-dependent Ca2+
channels in porcine
tracheal and bronchial smooth muscles. Anesthesiology 94:683, 2001.) Figure 6-3
Proposed signaling pathways underlying volatile anesthetic
(specifically halothane)-induced bronchodilation or inhibition of muscarinic agonist-induced
contraction of airway smooth muscle, or both mechanisms. Signal transduction involving
Rho protein is supported by work from Warner and coworkers on a role of halothane
to decrease Ca2+
sensitivity rather than a change in ICa2+
content. CaM, calmodulin; ICa2+
intracellular Ca2+
; IP3, inositol
triphosphate; MLC, myosin light chain; MLCK, myosin light chain kinase; pMLC, phosphorylated
myosin light chain; Rho, monomeric G protein; ROK, Rho-associated kinase; RyR, ryanodine
channels; SMPP, smooth muscle protein phosphatase; SR, sarcoplasmic reticulum; VDC,
voltage-dependent Ca2+
channels; encircled plus sign,
excitatory action of muscarinic receptor agonist; encircled
up arrow, represents activation or increase due to volatile anesthetic;
encircled down arrow, inhibition or decrease due
to volatile anesthetic. (Adapted from Hanazaki M, Jones KA, Perkins WJ,
et al: Halothane increases smooth muscle protein phosphatase in airway smooth muscle.
Anesthesiology 94:129, 2001 and from Pabelick CM, Prakash YS, Kannan MS, et al:
Effects of halothane on sarcoplasmic reticulum calcium release channels in porcine
airway smooth muscle cells. Anesthesiology 95:207, 2001.)
Figure 6-3
Proposed signaling pathways underlying volatile anesthetic
(specifically halothane)-induced bronchodilation or inhibition of muscarinic agonist-induced
contraction of airway smooth muscle, or both mechanisms. Signal transduction involving
Rho protein is supported by work from Warner and coworkers on a role of halothane
to decrease Ca2+
sensitivity rather than a change in ICa2+
content. CaM, calmodulin; ICa2+
intracellular Ca2+
; IP3, inositol
triphosphate; MLC, myosin light chain; MLCK, myosin light chain kinase; pMLC, phosphorylated
myosin light chain; Rho, monomeric G protein; ROK, Rho-associated kinase; RyR, ryanodine
channels; SMPP, smooth muscle protein phosphatase; SR, sarcoplasmic reticulum; VDC,
voltage-dependent Ca2+
channels; encircled plus sign,
excitatory action of muscarinic receptor agonist; encircled
up arrow, represents activation or increase due to volatile anesthetic;
encircled down arrow, inhibition or decrease due
to volatile anesthetic. (Adapted from Hanazaki M, Jones KA, Perkins WJ,
et al: Halothane increases smooth muscle protein phosphatase in airway smooth muscle.
Anesthesiology 94:129, 2001 and from Pabelick CM, Prakash YS, Kannan MS, et al:
Effects of halothane on sarcoplasmic reticulum calcium release channels in porcine
airway smooth muscle cells. Anesthesiology 95:207, 2001.)