|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The principles of intraoperative management of cardiothoracic surgical procedures are based on an understanding of the pathophysiology of each disease process and a working knowledge of the effects of the various anesthetic and other pharmacologic interventions on a particular patient's condition. Selecting an induction technique is dependent on the degree of cardiac dysfunction, the cardiac defect, the degree of sedation provided by the premedication, and the presence or absence of an indwelling catheter. In children with good cardiac reserve, induction techniques can be quite varied as long as induction is careful and well monitored. The titration of induction agents is more important than the specific anesthetic technique in patients with reasonable cardiac reserve. A wide spectrum of anesthetic induction techniques with a variety of agents has been used safely and successfully, such as sevoflurane, halothane, and nitrous oxide; intravenous and intramuscular ketamine; and intravenous propofol, fentanyl, midazolam, and thiopental.[63] For neonates undergoing open heart surgery, opioid-relaxant inductions are most prevalent, whereas older children with sufficient cardiac reserve typically receive inhalation inductions with either sevoflurane or halothane. The application of Emla cream (emulsion of lidocaine 2.5% and prilocaine 2.5%) at the site of intravenous cannula insertion facilitates cannulation and minimizes patient pain and stress. Ketamine has been the most popular agent for anesthetic induction in patients with cyanotic conditions because it increases systemic vascular resistance and cardiac output, thereby diminishing the magnitude of right-to-left shunting. Administration of ketamine can be intravenous or intramuscular. An intramuscular injection may result in pain, agitation, and subsequent arterial desaturation.
Inhalation inductions are generally well received and tolerated by most children. An inhalation induction with sevoflurane or halothane can easily and safely be performed even in cyanotic patients such as those with tetralogy of Fallot ( Fig. 51-7 ). In these patients, who are at risk for right-to-left shunting and systemic desaturation, oxygenation is well maintained with a good airway and ventilation, despite halothane-induced reduction in systemic arterial pressure.[64] Skilled airway management and efficiency of ventilation are equally essential components of anesthetic induction. Although it is essential to understand the complexities of shunts and vascular resistance changes, airway and ventilation effects on the cardiovascular system are of primary importance during the induction of anesthesia.
After anesthetic induction, intravenous access is established or augmented as appropriate. A nondepolarizing muscle relaxant is usually administered and an intravenous opioid and/or inhalation agent chosen for maintenance anesthesia. The child is preoxygenated with 100% FIO2 , and a lubricated nasal endotracheal tube is carefully positioned. A nasal tube is usually selected because most patients requiring an intraoperative TEE and/or postoperative mechanical ventilation, a nasotracheal tube provides greater stability and patient comfort
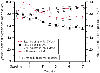 Figure 51-7
Comparative changes in arterial oxygen saturation (SaO2
)
and mean blood pressure (MAP) during mask halothane N2
O (n
= 7) and intramuscular ketamine (n = 7) inductions
in children with tetralogy of Fallot at risk for right-to-left shunting. Note the
maintenance of SaO2
in the halothane group
despite the significant drop in MAP. (From Greeley WJ, Bushman GA, Davis
DP, Reves JG: Comparative effects of halothane and ketamine on systemic arterial
oxygen saturation in children with cyanotic heart disease. Anesthesiology 65:666,
1986.)
Figure 51-7
Comparative changes in arterial oxygen saturation (SaO2
)
and mean blood pressure (MAP) during mask halothane N2
O (n
= 7) and intramuscular ketamine (n = 7) inductions
in children with tetralogy of Fallot at risk for right-to-left shunting. Note the
maintenance of SaO2
in the halothane group
despite the significant drop in MAP. (From Greeley WJ, Bushman GA, Davis
DP, Reves JG: Comparative effects of halothane and ketamine on systemic arterial
oxygen saturation in children with cyanotic heart disease. Anesthesiology 65:666,
1986.)
Because of the diverse array of congenital heart defects and surgical procedures, an individualized anesthetic management plan is essential. The maintenance of anesthesia in these patients depends on the age and condition of the patient, the nature of the surgical procedure, the duration of the CPB, and the need for postoperative ventilation. An assessment of the hemodynamic objectives designed to lessen the pathophysiologic loading conditions should be developed for each patient, taking advantage of the known qualitative effects of specific anesthetic agents and ventilatory strategies. These individualized plans must also be integrated with the overall perioperative goals to configure the optimal anesthetic. In patients with complex defects requiring preoperative inotropic and mechanical ventilatory support, a carefully controlled hemodynamic induction and maintenance anesthetic with a potent opioid is generally chosen. In patients with a simple ASD or VSD, a potent inhalation agent is preferred as the principal anesthetic agent. This allows for early postoperative extubation and a less prolonged period of intensive care monitoring. More important than the
The reported changes in blood pressure and heart rate for the inhalation agents in normal children are observed in pediatric cardiac surgical patients as well. Although both halothane and isoflurane decrease blood pressure in neonates, infants, and children, the vasodilatory properties of isoflurane may improve overall myocardial contractility, compared with the effects of halothane.[65] Despite improved cardiac reserve with isoflurane, the incidence of laryngospasm, coughing, and desaturation during induction of anesthesia limits its use as an induction agent in children with congenital heart defects.[66] The use of potent inhalation agents as primary anesthetics should be reserved for the child with adequate cardiovascular reserve who is a candidate for early postoperative extubation. In these patients, the myocardial depression and hypotension associated with the use of inhalation agents are well tolerated. Examples include closure of an ASD or VSD, excision of a discrete subaortic membrane, pulmonic or aortic stenosis, ligation of a PDA, and repair of coarctation of the aorta.
Desflurane has cardiorespiratory properties similar to those of isoflurane.[67] Its main advantage is low blood gas and tissue solubility. This allows for rapid equilibration between the inspired and alveolar concentrations and rapid decrease of alveolar concentrations during elimination.[68] This provides greater precision in drug dosing during the operative period and may make desflurane a more titratable adjunctive drug for pediatric cardiac anesthesia. The three main disadvantages of desflurane are potency, pungency, and negative inotropic effect.[69] [70] Studies in normal infants and children suggest that one minimum alveolar concentration of desflurane requires concentrations of 8% to 10%.[71] [72] Desflurane is also quite pungent and, although its uptake is rapid, early experience with this drug for inhalation induction in children has reported a fairly high incidence of airway reactivity and laryngospasm.[71] [73] [74] Although its negative inotropic effect is significantly less potent than that of halothane, desflurane should not be used as the sole anesthetic in patients with significant cardiac dysfunction.[74]
Sevoflurane offers a more tolerable aroma without the magnitude of myocardial depression that accompanies halothane.[75] In addition, its blood gas solubility is nearly as low as that of desflurane. Hemodynamically, sevoflurane tends to produce some tachycardia, particularly in older children, and preserve systemic arterial pressure.[76] Reductions in heart rate and systemic arterial pressure are more modest in infants anesthetized with sevoflurane than in control subjects anesthetized with halothane, and the former exhibit echocardiographic evidence of normal contractility and cardiac index.[77] [78] Controversies continue to surround the potential toxic byproducts of sevoflurane anesthesia, related both to patient metabolism and to the production of Compound A in the anesthesia breathing circuit. Although the importance of Compound A in adult practice remains uncertain, evidence suggests that production of this toxin is significantly diminished in children.[79]
Children with complex congenital heart disease and limited cardiac reserve demand an anesthetic technique that provides hemodynamic stability. Inhalation agents are less well tolerated as a primary anesthetic in patients who have limited cardiac reserve, especially after CPB. Fentanyl and sufentanil are excellent induction and maintenance anesthetics for this group of patients. Low to moderate doses of these opioids can be supplemented with inhalation anesthetics. Adding low concentrations of inhalation agents to smaller doses of opioids shortens or eliminates the need for postoperative mechanical ventilation while maintaining the advantage of intraoperative hemodynamic stability. Postoperative mechanical ventilation will be required when a high-dose opioid technique is used. The hemodynamic effects of fentanyl at a dose of 25 µg/kg with pancuronium given to infants in the postoperative period after operative repair of a congenital heart defect include no change in left atrial pressure, pulmonary artery pressure, pulmonary vascular resistance, and cardiac index and a small decrease in systemic vascular resistance and mean arterial pressure.[80] Higher doses of fentanyl at 50 to 75 µg/kg with pancuronium result in a slightly greater fall in arterial pressure and heart rate in infants undergoing repair for complex congenital heart defects.[81] Despite the wide safety margin exhibited by these opioids, a selected population of infants and children with marginally compensated hemodynamic function sustained by endogenous catecholamines may manifest more extreme cardiovascular changes with these doses. Fentanyl has also been shown to block stimulus-induced pulmonary vasoconstriction and contributes to the stability of the pulmonary circulation in neonates after congenital diaphragmatic hernia repair.[82] Thus, the use of fentanyl may be extrapolated to the operating room where stabilizing pulmonary vascular responsiveness in newborns and young infants with reactive pulmonary vascular beds is crucial to weaning from CPB and stabilizing shunt flow.
Sufentanil and pancuronium provide the same cardiovascular stability as fentanyl and pancuronium in pediatric cardiovascular patients. Children receiving a sufentanil induction as a single dose of 5 to 20 µg/kg have a stable preintubation period.[83] [84] Intubation and other stimuli such as sternotomy do not produce clinically significant alterations in hemodynamics, although changes are greater than with equipotent doses of fentanyl. The use of fentanyl as an infusion (.1 µg/kg/min) produces fewer alterations in heart rate and blood pressure. This is particularly important in infants in whom significant hemodynamic changes are poorly tolerated. For neonates with critical congenital heart disease, sufentanil anesthetic and postoperative infusion have been shown to reduce morbidity after cardiac surgery when compared with a halothane anesthetic and routine morphine postoperatively.[85] The blunting of the stress response observed in this study probably accounted for the differences in morbidity; there was no comparison group representing a more typical dose of a phenylpiperidine opioid (e.g., fentanyl, 0–75 µg/kg) to permit conclusions as to whether such large opioid doses are optimal.
| Age Group | t½a (min) | t½b (min) | Clearance (mL/kg/min) | Vdas (L/kg) |
|---|---|---|---|---|
| 1–30d | 23 ± 17 | 737 ± 346 | 6.7 ± 6.1 | 4.2 ± 1.0 |
| –24 mon | 16 ± 5 | 214 ± 41 | 18.1 ± 2.7 | 3.1 ± 1.0 |
| 2–12 y | 20 ± 6 | 140 ± 30 | 16.9 ± 2.2 | 2.7 ± 0.5 |
| 12–18 y | 20 ± 6 | 209 ± 23 | 13.1 ± 0.4 | 2.7 ± 0.5 |
| t½a, slow distribution half-life; t½b, elimination half-life; Vdas , volume of distribution at steady state. | ||||
Alfentanil is a short-acting potent opioid that has been used for cardiac surgery in children; it shows some promise in selected pediatric anesthesia cases because of its short elimination half-life and hemodynamic stability. As a primary anesthetic in children undergoing CPB, however, it must be continuously infused because of its short half-life. Also, when alfentanil infusion is discontinued, the release of stress hormones may increase at a more rapid rate compared with longer-acting opioids such as fentanyl. The use of alfentanil in congenital cardiac patients may therefore be limited to simple repairs such as an ASD, in which the bypass time is short and temperature extremes are less severe, and prompt tracheal extubation is a goal.
Compared with other agents in its class, remifentanil, a new ultrashort-acting opioid, offers the unique advantage of metabolism via nonspecific and tissue esterases, thereby limiting the potential for accumulation related to protracted elimination. [86] Remifentanil may provide the advantages of alfentanil in the selected group for whom the blunting of endogenous responses is desirable intraoperatively, but potentially deleterious at the end of the procedure. A randomized controlled trial comparing equipotent doses of alfentanil and remifentanil for outpatient pediatric surgery revealed delayed emergence, requiring naloxone only in the alfentanil group.[87] In both adults and children, remifentanil is associated with qualitative hemodynamic changes similar to other opioids, a variable tendency to bradycardia, and a small reduction in systolic blood pressure.[87] [88] [89]
Because of the widespread use of the opioids for pediatric cardiac surgery and the availability of invasive monitoring, the pharmacokinetics and pharmacodynamics of these drugs have been well studied.[84] [90] In general, the clinical pharmacology of fentanyl and sufentanil share the same age-related pharmacokinetic and pharmacodynamic features. For example, sufentanil has increased clearance in patients aged 1 month to 12 years, comparable adult clearance in adolescents (12 to 16 years of age), and decreased clearance during the neonatal period (newborn to 1 month) ( Table 51-6 ).[73] [78] Furthermore, sequential sufentanil anesthetics in neonates with congenital heart disease show marked increases in clearance and elimination between the 1st week and the 3rd or 4th week of life[79] ( Fig. 51-8 ). The latter observation is most likely attributable to maturational changes in hepatic microsomal activity and improved hepatic blood flow from closure of the ductus venosus. The variability in clearance and elimination, coupled with limited cardiovascular reserve in the neonate during the first month of life, makes opioid dosing difficult in this age group. Careful titration of 5 to 10 µg/kg of fentanyl or 1 to 2 µg/kg of sufentanil or a continuous infusion technique provides the most reliable method of achieving hemodynamic stability and an accurate dose response. Cardiopulmonary bypass, different institutional anesthetic practices, and individual patient differences influence pharmacokinetic and pharmacodynamic disposition of the opioids in ways that are not predictable. Even certain disease states such as tetralogy of Fallot[80] or pathophysiologic conditions such as increased intra-abdominal pressure[81] alter pharmacokinetic processes.
|
|
|
|
|
|
|
|
|
|
|
|
|