|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The physiologic effects of CPB on neonates, infants, and children are significantly different than the effects on adults ( Table 51-7 ). During CPB, pediatric patients are exposed to biologic extremes not seen in adults, including deep hypothermia (18°C), hemodilution (three- to fivefold greater dilution of circulating blood volume), low perfusion pressures (20 to 30 mm Hg), wide variation in pump
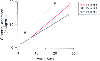 Figure 51-8
Sequential sufentanil clearance during the first month
of life in three neonates with congenital heart disease. Clearance of sufentanil
increases above adult rates within the neonatal period. (Data from Greeley
WJ, de Bruijn NP: Changes in sufentanil pharmacokinetics within the neonatal period.
Anesth Analg 67:86, 1988.)
Figure 51-8
Sequential sufentanil clearance during the first month
of life in three neonates with congenital heart disease. Clearance of sufentanil
increases above adult rates within the neonatal period. (Data from Greeley
WJ, de Bruijn NP: Changes in sufentanil pharmacokinetics within the neonatal period.
Anesth Analg 67:86, 1988.)
| Parameter | Adult | Pediatric |
|---|---|---|
| Hypothermic temperature | Rarely below 25–30°C | Commonly 15–20°C |
| Use of total circulatory arrest | Rare | Common |
| Pump prime |
|
|
| Dilution effects on blood volume: additional additives in pediatric primes | 25–33% | 150–300% |
|
|
|
Blood, albumin |
| Perfusion pressures | 50–80 mm Hg | 20–50 mm Hg |
| Influence of α- vs. pH-stat management strategy | Minimal at moderate hypothermia | Marked at deep hypothermia |
| Measured PaCO2 differences | 30–45 mm Hg | 20–80 mm Hg |
| Glucose regulation |
|
|
| Hypoglycemia | Rare—requires significant hepatic injury | Common—reduced hepatic glycogen stores |
| Hyperglycemia | Frequent—generally easily controlled with insulin | Less common—rebound hypoglycemia may occur |
Adult patients are infrequently exposed to these biologic extremes. In adult cardiac patients, temperature is rarely lowered below 25°C, hemodilution is more moderate, perfusion pressure is generally maintained at 50 to 80 mm Hg, flow rates are maintained at 50 to 65 mL/kg/min, and pH management strategy is less influential because of moderate hypothermic temperatures and rare use of circulatory arrest. Variables such as glucose supplementation rarely pose a problem in adult patients owing to large hepatic glycogen stores. Venous and arterial cannulas are larger and less deforming of the atria and aorta, and their placement is more predictable. Although superficially similar, the conduct of CPB in children is considerably different from that in adults. One would therefore expect marked physiologic differences in the response to CPB in children.
The priming solutions used in pediatric CPB take on great importance because of the disproportionately large priming volume/blood volume ratio in children. In adults, the priming volume is equivalent to 25% to 33% of the patient's blood volume, whereas in neonates and infants, the priming volume may exceed the patient's blood volume by 200%. With contemporary low-volume bypass circuits (small volume oxygenators, smaller tubing, etc.) priming volume is not more than one blood volume in a small neonate. Care must be taken, therefore, to achieve a physiologically balanced prime and limit the volume as much as possible. Most pediatric priming solutions, however, have quite variable levels of electrolytes, calcium, glucose, and lactate. Electrolytes, glucose, and lactate levels may be quite high if the prime includes large amounts of banked blood, or quite low if a minimal amount of banked blood is added. Calcium levels are generally very low in pediatric priming solutions; this may contribute to the rapid slowing of the heart with the initiation of bypass.
The main constituents of the priming solution include crystalloid, banked blood (to maintain a temperature-appropriate hematocrit), and colloid. Other supplements that may be added to the prime are mannitol, a buffer (sodium bicarbonate or trishydroxymethylaminomethane [THAM]), and steroids. Many institutions add colloid or fresh frozen plasma to the pump prime in neonates and small infants or use whole blood in the priming solution. Low concentrations of plasma proteins have been shown experimentally to impair lymphatic flow and alter pulmonary function by increasing capillary leak.[94] [95] Although adding albumin to the pump prime has not been shown to alter outcome in adults during CPB, one study suggested that maintaining normal colloid osmotic pressure may improve survival in infants undergoing CPB.[96] [97]
The addition of fresh frozen plasma or whole blood is an attempt to restore the level of procoagulants, which are severely diluted with CPB in infants. For neonates and infants, blood must be added to the priming solution. Most institutions use packed RBCs, but some use whole blood. The use of whole blood supplements both RBCs and the coagulation factors with a single donor exposure. In fact, low- volume bypass circuits may enable perfusionists and anesthesiologists to share a single unit of whole blood, thereby limiting the donor exposure to one throughout the entire perioperative course.
The addition of any blood products will cause a much higher glucose load in the prime. Hyperglycemia may increase the risk of neurologic injury if brain ischemia occurs. Mannitol is added to promote an osmotic diuresis and to scavenge oxygen free radicals from the circulation. Steroids are added to stabilize membranes to produce the theoretical advantage of reducing ion shifts during periods of ischemia. Steroids, however, may raise glucose levels and this may be detrimental if there is a period of cerebral ischemia. Steroids remain one of the more controversial additives in priming solutions.
Hypothermic CPB is used to preserve organ function during cardiac surgery. Three distinct methods of CPB are used: moderate hypothermia (25 to 32°C), deep hypothermia (18°C), and DHCA. The choice of method of bypass is based on the required surgical conditions, patient size, the type of operation, and the potential physiologic impact on the patient.
Moderate hypothermic CPB is the principal method of bypass employed for older children and adolescents. In these patients, venous cannulas are less obtrusive, and the heart can easily accommodate superior and inferior vena cava cannulation. Bicaval cannulation reduces right atrial blood return and improves the surgeon's ability to visualize intracardiac anatomy. Moderate hypothermia may also be chosen for less demanding cardiac repairs in infants, such as an ASD or an uncomplicated VSD. Most surgeons are willing to cannulate the inferior and superior venae cavae in neonates and infants. In these patients, however, this approach is technically more difficult and likely to induce brief periods of hemodynamic instability. Additionally, the pliability of the cavae and the rigidity of the cannulas may result in caval obstruction, impaired venous drainage, and elevated venous pressure in the mesenteric and cerebral circulation.
Deep hypothermic CPB is generally reserved for neonates and infants requiring complex cardiac repair. However, certain older children with complex cardiac disease or severe aortic arch disease benefit from deep hypothermic temperatures. For the most part, deep hypothermia is selected to allow the surgeon to operate under conditions of low-flow CPB or total circulatory arrest. Low pump flows (50 mL/kg/min) improve the operating conditions for the surgeon by providing a nearly bloodless field. DHCA allows the surgeon to remove the atrial or aortic cannula. Utilizing this technique, surgical repair is more precise because of the bloodless and cannula-free operative field. Arresting the circulation, even at deep hypothermic temperatures, introduces the concern of how well deep hypothermia preserves organ function, with the brain being at greatest risk.[98]
Although hemoconcentrated blood has an improved oxygen-carrying capacity, its viscosity reduces efficient flow through the microcirculation. With hypothermic temperatures, blood viscosity increases significantly and flow decreases. Hypothermia, coupled with the nonpulsatile flow of CPB, impairs blood flow through the microcirculation. Blood sludging, small vessel occlusion, and multiple areas of tissue hypoperfusion may result. Therefore, hemodilution is an important consideration during hypothermic CPB. The appropriate level of hemodilution for a given hypothermic temperature, however, is not well defined. Experimental evidence suggests that reducing hematocrit levels to as low as 15% provides a sufficient quantity of oxygen delivery to the myocardium at normothermia, provided that intravascular volume, colloid osmotic pressure, and normotension are maintained.[99] At hypothermic temperatures, hematocrit levels reduced to as low as 15% provide adequate oxygen delivery during CPB, as long as flow rates and perfusion pressure are maintained. [100] [101] Because RBCs serve as the major reservoir of oxygen during circulatory arrest, especially during rewarming, hematocrit values closer to 30% are generally preferred for deep hypothermia when this technique is contemplated. Currently, most centers maintain hematocrit levels around 25% to 30% during CPB, enhancing oxygen delivery to vital organs such as the brain. Cerebral oxygen delivery is an especially important consideration, because cerebral autoregulation is impaired at deep hypothermic temperatures and after DHCA.
To achieve a hematocrit level of 25% to 30% in neonates and infants,
banked blood should be added to the priming solution. A calculation of the mixed
hematocrit level on CPB (the hematocrit level of the total priming volume plus the
patient's blood volume) can be calculated by the following formula:
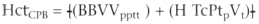
where HctCPB
is the mixed hematocrit (TPV + BVpt
), BVpt
is the patient's blood volume (weight in kg × estimated blood volume in mL/kg),
TPV is the total priming volume, and Hctpt
is the starting hematocrit
level of the patient. This calculation allows an estimate of the hematocrit level
of the patient using an asanguineous prime and is therefore useful for older children
and adolescents. In neonates and infants, the perfusionist must add blood to the
pump prime to achieve a desired hematocrit level during hypothermic CPB. The following
formula estimates the amount of RBCs that must be added to achieve this hematocrit
level:
Added RBCs (mL) = (BVpt
+ TPV)(Hctdesired
)
− (BVpt
)(Hctpt
)
where added RBCs represents milliliters of packed RBCs added to the prime volume,
BVpt
is the patient's blood volume, TPV is the total priming volume, Hctdesired
is the desired hematocrit level on CPB, and Hctpt
is the starting hematocrit
level of the patient.
Currently, no evidence exists for defining the optimal hematocrit level after weaning from CPB. Decisions concerning post-CPB hematocrit levels are made based on the patient's post-repair function and anatomy. Patients with residual hypoxemia or those with moderate to severe myocardial dysfunction benefit from the improved oxygen-carrying capacity of hematocrit levels of 40% or higher. Patients with a physiologic correction and excellent myocardial function may tolerate hematocrit levels of 25% to 30%.[99] In children with mild to moderate myocardial dysfunction, accepting hematocrit values between these levels seems prudent. Therefore, in patients with physiologic correction, moderately good ventricular function, and hemodynamic stability, the risks associated with blood and blood product transfusion should be strongly considered during the immediate post-bypass period.
|
|
|
|
|
|
|
|
|
|
|
|
|