|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In general, the renal response to any specific condition or insult is primarily determined by associated physiologic and hemodynamic conditions prevailing at the time (see Chapter 20 ). In patients with renal disease, the challenge of optimal perioperative management is demanding because recognition of subtle change is difficult. Renal failure symptoms typically are not detected until less than 40% of normal functioning nephrons remain, and uremic symptoms occur when less than 5% of normal functioning nephrons remain. [18]
The three general functions of the kidneys are to excrete potentially toxic metabolic end products, to regulate water and tonicity, and to produce hormones. The kidney excretes most of the waste products metabolized from dietary intake, chiefly urea formed from ammonia released in protein metabolism, creatinine from muscle metabolism, and uric acid from purine metabolism. Serum creatinine levels and blood urea nitrogen (BUN) levels are used to measure the efficiency of metabolic waste removal. The levels of these end products rise slightly with increased dietary intake or increased catabolism. With normal renal function, an increase in concentration of waste products in blood results in rapid excretion of the increased load. The waste products are filtered in the glomeruli and tend to remain in the tubular fluid because they cannot permeate the nephron wall. In contrast, more than 98% of the filtered water and solute is reabsorbed into the body. Some degradation products, such as uric acid, are reabsorbed in the proximal tubule, secreted back into the tubule fluid, and then eliminated in urine. Cells and proteins with molecular masses greater than 70,000 daltons do not cross the glomerular capillary wall. However, smaller proteins, peptides, amino acids, and glucose are filtered to a variable degree. The proximal
 Figure 37-1
The glomerular permeability (i.e., filtration-to-plasma
ratio) is represented on the y axis and compared
with the molecular weight of the filtered substance represented on the x
axis. (Data from Renkin EM, Robinson RR: Glomerular filtration. N Engl
J Med 290:785, 1974.)
Figure 37-1
The glomerular permeability (i.e., filtration-to-plasma
ratio) is represented on the y axis and compared
with the molecular weight of the filtered substance represented on the x
axis. (Data from Renkin EM, Robinson RR: Glomerular filtration. N Engl
J Med 290:785, 1974.)
The kidney regulates extracellular volume (approximately one third of total body water) primarily by manipulating sodium to maintain normal tonicity. The body
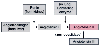 Figure 37-2
Renin released into the circulation interacts with angiotensinogen
to cleave off the decapeptide angiotensin I; through converting enzymatic activity,
this becomes the active octapeptide angiotensin II. Angiotensin II is a potent vasopressor
and has a direct effect on the adrenals to stimulate aldosterone. By its vasoconstrictive
action, angiotensin II increases salt retention.
Figure 37-2
Renin released into the circulation interacts with angiotensinogen
to cleave off the decapeptide angiotensin I; through converting enzymatic activity,
this becomes the active octapeptide angiotensin II. Angiotensin II is a potent vasopressor
and has a direct effect on the adrenals to stimulate aldosterone. By its vasoconstrictive
action, angiotensin II increases salt retention.
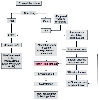 Figure 37-3
Mechanism for renal sodium and volume regulation. ANF,
atrial natriuretic factor; B.P., blood pressure; C.O., cardiac output; GFR, glomerular
filtration rate; NaCl, sodium chloride.
Figure 37-3
Mechanism for renal sodium and volume regulation. ANF,
atrial natriuretic factor; B.P., blood pressure; C.O., cardiac output; GFR, glomerular
filtration rate; NaCl, sodium chloride.
The second part of the tonicity function that the kidney serves is maintaining osmolarity within normal limits through regulation of water excretion. Reduced water intake with continued urinary water excretion leads to a rise in plasma osmolarity and vice versa. The major effector in the regulation of water excretion is the antidiuretic hormone (ADH) vasopressin. Vasopressin is secreted into the peripheral plasma by the posterior pituitary gland. The kidney is capable of wide variations in water excretion (i.e., urine flow) in response to changing levels of vasopressin in the peripheral plasma. Water excretion can be 100-fold lower in extreme antiduresis (i.e., high vasopressin) than in extreme diuresis. These changes are achieved without substantial changes in steady-state rate of total solute excretion (i.e., osmolar excretion). This capability depends on the kidneys' ability to concentrate or dilute the urine. Water output from the kidneys of a 70-kg person normally ranges from 500 mL to 12 L/day. The kidney maintains a normal range of urinary concentrations and volumes first by producing a large amount of dilute fluid in the loop of Henle and the distal convoluted tubule. When the fluid passes through the highly concentrated solutes in the medulla in the presence of ADH, most of the water is reabsorbed. In the absence of ADH, only about one half of the water is reabsorbed. An acute (transient) effect of altered levels of circulating vasopressin (or ADH) is different from a steady-state response. Such transient behavior is observed in response to altered levels of circulating vasopressin and after induction of anesthesia.
The medullary concentration of solutes is high (1400 mOsm/L) because the ascending limb of the loop of Henle actively reabsorbs sodium and chloride without water into the medullary interstitium and because large amounts of urea enter the inner medulla from the collecting tubule. The cortical and outer medullary collecting tubules are sites of passive water reabsorption that are impermeable to urea. Urea becomes concentrated in the collecting ducts of the inner medulla, which are permeable to urea moving passively down its concentration gradient. The high concentrations of sodium, chloride, and urea are not washed out of the medulla because medullary blood flow is low and the configuration of the capillaries of the vasa recta permits a countercurrent exchange. Of the 140 to 180 L/day of fluid filtered at glomeruli, approximately 70% (100 L/day) is reabsorbed isosmotically in the proximal tubule, and 15% (20 L/day) is reabsorbed passively in the medulla in the descending loop of Henle. The remaining 15% (20 L/day) of highly concentrated tubular fluid reaches the papillary tip of the loop of Henle. As the fluid ascends the loop of Henle, sodium is reabsorbed (passively in the medulla and actively in the thick ascending limb in the outer medulla and cortex). Tubular fluid becomes hypotonic to plasma. Sodium reabsorption without water continues in the distal tubule. The collecting ducts may receive tubular fluid with concentrations below 100 mOsm/L. Tubular permeability to water increases or decreases, depending on the amount of ADH present, and may vary from 8 to 20 L, with subsequent urine volumes varying from 0.5 to 12 L/day in the normal 70-kg person.
The kidney functions as an endocrine gland in the renin-angiotensin system. Renin is secreted into the body by the granular cells of the juxtaglomerular apparatus of the kidney. Once in the bloodstream, renin catalyzes the splitting of angiotensin I from angiotensinogen, which is secreted from the liver and is always present in the plasma in high concentrations. The kidneys also secrete renal erythropoietic factor, which is involved in the control of erythrocyte production by the bone marrow. In response to decreased oxygen delivery to the kidney, renal erythropoietic factor is secreted and acts enzymatically in the plasma on a globulin secreted by the liver to form erythropoietin, which then acts to stimulate the bone marrow to increase its production of erythrocytes. The kidneys produce the active form of vitamin D and are linked to calcium metabolism; during times of prolonged fasting, the kidneys synthesize glucose from amino acids and other precursors and have the potential to be gluconeogenic organs.
Urine formation depends on hydraulic and oncotic pressures within the glomerular capillary, local and systemic neurohumoral influences, and an intact kidney-ureter-bladder feedback loop (see Chapter 20 ). The formation of urine begins with glomerular ultrafiltration and progresses to selective tubular reabsorption and tubular secretion. General anesthesia and surgery influence renal function primarily by affecting filtration and reabsorption. [21] [22] [23] [24] [25] All anesthetic agents, whether volatile or intravenous, have the potential to alter renal function by altering blood pressure and cardiac output so that renal blood flow is redistributed. This redistribution is accompanied by sodium and water conservation and decreased urine formation. Commonly used premedications can also influence urine output.[26] Narcotics and barbiturates, for example, can produce a small decrease in glomerular filtration rate (GFR) and, consequently, urine volume.
Regional anesthesia above level T4 reduces sympathetic tone to the kidney and makes renal blood flow and filtration directly dependent on perfusion pressure during sympathectomy.[27] Renal function and urine formation during regional anesthesia depend on the overall sum of the different homeostatic responses evoked, not just the sympathectomy. The combined effect of multiple factors, such as level of the block, preservation of autoregulatory mechanisms to preserve renal blood flow, circulating catecholamines, renin-angiotensin, ADH,
Anesthetic drugs may increase or decrease catecholamines, alter renal vascular resistance, depress myocardium, and decrease renal blood flow, or they may have a direct nephrotoxic effect on renal tubular function. Surgery in general, aortic cross-clamping and declamping in particular, trauma, and stress also may indirectly influence urine formation by changing myocardial function, sympathetic activity, neuronal or hormonal activity (e.g., renin and angiotensin production), intravascular volume, or systemic vascular resistance.[28] [29]
Glomerular filtration is governed by the following equation:
GFR = Kf
× (PGC
− PBC − PPO)
GFR is the glomerular filtration rate or the rate at which fluid is filtered through
the glomerular capillaries into the Bowman capsule, Kf
is the glomerular
filtration coefficient or the factor that considers the permeability and surface
area of the glomerular basement membrane, PGC is
the glomerular capillary pressure, PBC is the pressure
in the Bowman capsule, and PPO is the plasma oncotic
pressure.
The last three factors represent the Starling forces that govern the hydrostatic forces through the glomerular membrane. Acute changes in GFR, and consequently in urine formation, are commonly caused by changes in glomerular capillary pressure. For example, events that reduce plasma flow rates reduce the hydraulic pressure of glomerular capillaries and favor decreased ultrafiltration, whereas events that increase plasma flow rate have the opposite effect. Theoretically, varying concentrations of plasma proteins may influence urine formation and the GFR. As the concentration of proteins in plasma decreases, the oncotic pressure and the oncotic force opposing ultrafiltration also decrease. Conversely, a rise in oncotic pressure reduces the rate of glomerular filtration. This effect is most likely offset by the oncotic force of plasma proteins (e.g., albumin), which enables adequate intravascular volume to be maintained. The precise role in preserving renal function of oncotic pressure versus maintenance of adequate renal blood flow or other variables influencing glomerular filtration has never been studied. However, in one series,[30] patients resuscitated with albumin after massive transfusion had more renal dysfunction than those resuscitated with saline.
When the impact of normal saline-based intravenous fluid replacement was evaluated and compared with lactated Ringer's solution (i.e., albumin 5% in normal saline, hetastarch in normal saline, or lactated Ringer's solution), renal function indices (i.e., creatinine clearance, serum creatinine, and urine output) were all inferior in the normal saline-based vehicle groups.[31] The patients receiving lactated Ringer's solution were more likely to exhibit a hypercoagulable state.[31] Outcome data, however, did not enable a distinction between albumin and normal saline as a fluid replacement option.
Because the tubules and the collecting ducts of the kidney reabsorb about 99% of the filtered solute, the daily filtration volume (approximately 180 L) greatly exceeds the typical daily urinary volume of 1 to 2 L. To clear nitrogenous wastes daily, 400 to 500 mL of urine is required. The countercurrent multiplier system in the loop of Henle is a critical component of the kidney's ability to excrete or conserve salt and water. Sodium and water reabsorption depend on the hypertonicity of the medullary interstitium, which depends on the maintenance of normal renal blood flow. The major hormonal influences determining conservation or loss of filtered sodium and water are aldosterone, ADH, atrial natriuretic factor, and the renal prostaglandins.
High concentrations of aldosterone stimulate reabsorption of sodium and water, primarily in the distal tubule and the collecting ducts. Aldosterone is produced by the adrenal cortex in response to the feedback from the reninangiotensin-aldosterone system, simplified as follows. Reduced delivery of sodium to the macula densa causes release of renin from the granular cells of the juxtaglomerular apparatus. Renin catalyzes the release of angiotensin I from angiotensinogen. Angiotensin I is then transformed to angiotensin II in the lungs, catalyzed by angiotensin-converting enzyme. Angiotensin II stimulates the production of aldosterone.
ADH acts primarily on the collecting ducts to increase water reabsorption. ADH is released from the posterior pituitary gland in response to increased blood osmolarity, which stimulates osmoreceptors in the hypothalamus. ADH is inhibited by stimulation of atrial baroreceptors or increased atrial volume.[32] ADH release is also influenced by stress and increased partial pressure of arterial carbon dioxide.[33] A high level of ADH results in the excretion of small volumes of concentrated urine.
Atrial natriuretic peptide causes systemic vasodilation and promotes renal excretion of sodium and water by increasing glomerular filtration.[34] Atrial natriuretic peptide is secreted by the cardiac atria and other organs in response to increased intravascular volume. It decreases systemic blood pressure by relaxing vascular smooth muscle, reducing sympathetic stimulation, and inhibiting the renin-angiotensin-aldosterone system.
The kidney also synthesizes prostaglandins, which regulate the influence of other hormones. During hemodynamic instability and increased adrenergic stimulation, for example, prostaglandin E2 decreases the vasoconstrictive effect of angiotensin II on the afferent arterioles and preserves renal blood flow. Inhibition of prostaglandin synthesis during normal states of hydration, renal perfusion, and sodium balance does not affect renal function. When the kidney is confronted with a vasoconstricted state, such as hypotension and hypovolemia, however, the presence of renal prostaglandins is essential for preserving adequate renal blood flow. Patients taking non-steroidal anti-inflammatory drugs are at risk during conditions of impaired circulatory status because these agents inhibit cyclooxygenase activity, an important enzyme in the prostaglandin synthesis pathway, thereby rendering the kidney (afferent arteriole) susceptible to the systemic vasoconstrictive effect of angiotensin II and
Ureteral peristalsis influences urine formation. Interaction between the urinary bladder, the kidney, and the ureters regulates urine production by the kidney and urine transport to the bladder by the ureter.[35] General anesthesia decreases the rate of ureteral contractility ( Fig. 37-4 ).[36] Normally, ureteral peristalsis originates with electrical activity at pacemaker sites located in the proximal portion of the urinary collecting system. The electrical activity is then propagated, giving rise to the mechanical event peristalsis and ureteral contraction, which propels the bolus of urine distally. Basal peristalsis within the ureter is influenced by pressures within the renal pelvis, the ureter, and the ureteral-vesical junction. The autonomic nervous system also plays an important role in ureteral function and consequently in urine formation.[37] Cholinergic agonists generally increase the frequency and the force of ureteral contraction. Drugs that act primarily on adrenergic receptors tend to stimulate ureteral activity, whereas agents that primarily activate adrenergic receptors tend to inhibit ureteral activity. Histamine stimulates ureteral activity. In a variety of preparations, morphine increases ureteral tone. Infections within the urinary tract may impair urine transport. In humans, irregular peristaltic contractions occur with retroperitoneal inflammation resulting from appendicitis, regional enteritis, ulcerative colitis, and peritonitis.[38] Clinically, the response of the ureter to pathologic conditions seems to vary with age. More marked degrees of ureteral dilation are observed in neonates and young children than in adults. [39] Aging brings a decrease in the effectiveness of β-adrenergic agonists to relax the ureter and a progressive increase in smooth muscle cell mass, producing the overall effect of no apparent change in the ureteral contractile process in the geriatric patient.
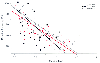 Figure 37-4
Effect of general anesthesia on the rate of ureteral
contractility. (Adapted from Young CJ, Attele A, Toledano A, et al: Volatile
anesthetics decrease peristalsis in the guinea pig ureter. Anesthesiology 81:452–458,
1994.)
Figure 37-4
Effect of general anesthesia on the rate of ureteral
contractility. (Adapted from Young CJ, Attele A, Toledano A, et al: Volatile
anesthetics decrease peristalsis in the guinea pig ureter. Anesthesiology 81:452–458,
1994.)
|
|
|
|
|
|
|
|
|
|
|
|
|