|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The kidneys contain approximately 2 × 106 nephrons, each of which consists of a glomerulus and a tubule, which empties into a collecting duct. These functional units collectively enable the kidneys to maintain a remarkably stable interior milieu despite large fluctuations in fluid and solute intake. Together, they regulate intravascular volume, osmolality, and acid-base and electrolyte balance and excrete the end products of metabolism and drugs. Urine is formed by the combination of glomerular ultrafiltration and tubular reabsorption and secretion. The nephron also elaborates hormones that contribute to fluid homeostasis (renin, prostaglandins, kinins), bone metabolism (1,25-dihydroxycholecalciferol), and hematopoiesis (erythropoietin). The function of the nephron is closely integrated with the vascular supply of the kidney ( Fig. 20-1 ).
The glomerulus consists of five distinct components: capillary endothelium, glomerular basement membrane, visceral epithelium (which together make up the filtration barrier), parietal epithelium (Bowman's capsule), and mesangium (interstitial cells).[1] [2] The glomerular tuft, a highly convoluted series of capillary loops, is fed by the afferent arteriole and drains into the efferent arteriole ( Fig. 20-2 ).
The capillary endothelium has fenestrations about 70 to 100 nm in diameter and lies atop the glomerular basement membrane, which has a total cross section of about 350 nm. The visceral epithelium, which is applied to the underside of the basement membrane, consists of podocytes with filamentous, interdigitating foot processes that contain contractile actin filaments. Filtration slits form 25- to 60-nm gaps between the foot processes and are bridged by a membranous slit diaphragm, whose size and permeability are altered by contraction of the foot processes.
The blind parietal epithelial sac of the renal tubule is invaginated around the capillary tuft as Bowman's capsule and meets the visceral epithelium at the vascular pole of the glomerulus. Bowman's space, between the visceral and parietal layers of the capsule, becomes the lumen of the proximal tubule at the urinary pole of the glomerulus, and the parietal endothelium merges with the cuboidal cells of the proximal tubule.
The central or interstitial mesangial cells are specialized pericytes with numerous functions, including structural support, matrix elaboration, and phagocytosis. They contain myofilament-like threads of actin and myosin. Mesangial contraction in response to vasoactive substances such as angiotensin II restricts blood flow to fewer capillary loops. The mesangial cells thereby regulate the
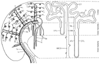 Figure 20-1
Anatomic relationships of the nephron and the renal vasculature.
The left side of the diagram represents the renal
vasculature as distributed through the inner medulla, outer medulla, and cortex.
Arteries are drawn as solid lines, veins as hollow
tubes. The renal artery divides serially into interlobar arteries (1),
arcuate arteries (2), and interlobular arteries (3). The afferent arterioles (5)
branch off laterally and provide the capillary tufts of the renal glomeruli in the
outer cortex (7a), whose efferent arterioles (6) supply the cortical capillary network
(not shown). In the juxtamedullary zone (7b), the efferent arterioles become the
vasa recta, which are closely applied to the long loops of Henle (8, 8a, 9). The
venous drainage consists of stellate veins (4), interlobular veins (3a), arcuate
veins (2a), and interlobar veins (1a). The right
side of the diagram represents two nephrons. On the left
is the more numerous superficial cortical nephron with a short loop of Henle. On
the right is the juxtamedullary nephron with a long
loop of Henle, which dives deep into the inner medulla to generate the hyperosmotic
interstitium required for tubular urine concentration. ATL, ascending thin loop
of Henle; CCD, cortical collecting duct; DT, distal tubule; DTL, descending thin
loop of Henle; G, glomerulus; IMCD, inner medullary collecting duct; OMCD, outer
medullary collecting duct; PT, proximal tubule; TAL, thick ascending loop. (Used
with permission from Kriz W: A standard nomenclature for structures of the kidney.
Kidney Int 33:1–7, 1988.)
Figure 20-1
Anatomic relationships of the nephron and the renal vasculature.
The left side of the diagram represents the renal
vasculature as distributed through the inner medulla, outer medulla, and cortex.
Arteries are drawn as solid lines, veins as hollow
tubes. The renal artery divides serially into interlobar arteries (1),
arcuate arteries (2), and interlobular arteries (3). The afferent arterioles (5)
branch off laterally and provide the capillary tufts of the renal glomeruli in the
outer cortex (7a), whose efferent arterioles (6) supply the cortical capillary network
(not shown). In the juxtamedullary zone (7b), the efferent arterioles become the
vasa recta, which are closely applied to the long loops of Henle (8, 8a, 9). The
venous drainage consists of stellate veins (4), interlobular veins (3a), arcuate
veins (2a), and interlobar veins (1a). The right
side of the diagram represents two nephrons. On the left
is the more numerous superficial cortical nephron with a short loop of Henle. On
the right is the juxtamedullary nephron with a long
loop of Henle, which dives deep into the inner medulla to generate the hyperosmotic
interstitium required for tubular urine concentration. ATL, ascending thin loop
of Henle; CCD, cortical collecting duct; DT, distal tubule; DTL, descending thin
loop of Henle; G, glomerulus; IMCD, inner medullary collecting duct; OMCD, outer
medullary collecting duct; PT, proximal tubule; TAL, thick ascending loop. (Used
with permission from Kriz W: A standard nomenclature for structures of the kidney.
Kidney Int 33:1–7, 1988.)
To cross the filtration barrier between plasma and tubular fluid, a molecule must pass in succession through the endothelial fenestrations, the glomerular basement membrane, and the epithelial slit diaphragm. The capillary endothelium restricts the passage of cells, but the basement membrane filters plasma proteins. All three layers contain negatively charged glycoproteins that retard the passage of other negatively charged proteins. Thus, the filtration barrier is both size and charge selective.[2] Molecules with an effective radius of less than 1.8 nm (e.g., water, sodium, urea, glucose, inulin) are freely filtered. Molecules larger than 3.6 nm (e.g., hemoglobin, albumin) are not filtered. Filtration of molecules between 1.8 and 3.6 nm depends on their electrical charge. Cations are filtered, whereas anions are not. In glomerulonephritis, the negatively charged glycoproteins are destroyed, polyanionic proteins are filtered, and proteinuria ensues.
Glomerular ultrafiltration is governed by the balance of Starling's
forces regulating fluid flux across the filtration barrier.[2]
The glomerular filtration rate (GFR) depends on the permeability of the filtration
barrier and the net difference between hydrostatic forces pushing fluid into Bowman's
space and osmotic forces keeping fluid in plasma:
GFR = Kuf
[(Pgc
− Pbs
)
− (πgc
− πbs
)] (1)
where uf = ultrafiltration, gc = glomerular capillary, and bs = Bowman's space.
The ultrafiltration coefficient Kuf reflects capillary permeability and the glomerular surface area. Renal arterial pressure determines the hydrostatic pressure in the glomerular capillary (Pgc ). Afferent arteriolar plasma flow determines plasma oncotic pressure (πgc ): rapid blood flow washes out osmotically effective molecules and lowers πgc , and vice versa.
The juxtaglomerular apparatus provides a remarkable integration of tubular and glomerular structure and function ( Fig. 20-3 ). A modified portion of the thick ascending limb, the macula densa, is applied to the glomerulus
 Figure 20-2
Photomicrograph of a cast of a glomerulus without Bowman's
capsule. At the lower left, the afferent arteriole
(A) originates from an interlobular artery and enters the glomerulus with its many
capillary loops. At the upper left, the efferent
arteriole (E) leaves the glomerulus and branches to form the peritubular capillary
plexus (magnification ×300). (From Tisher CC, Madsen KM: Anatomy
of the kidney. In Brenner BM [ed]: Brenner &
Rector's The Kidney, 6th ed. Philadelphia, WB Saunders, 2000, pp 3–67.)
Figure 20-2
Photomicrograph of a cast of a glomerulus without Bowman's
capsule. At the lower left, the afferent arteriole
(A) originates from an interlobular artery and enters the glomerulus with its many
capillary loops. At the upper left, the efferent
arteriole (E) leaves the glomerulus and branches to form the peritubular capillary
plexus (magnification ×300). (From Tisher CC, Madsen KM: Anatomy
of the kidney. In Brenner BM [ed]: Brenner &
Rector's The Kidney, 6th ed. Philadelphia, WB Saunders, 2000, pp 3–67.)
A primary determinant of the GFR is glomerular filtration pressure, which depends not only on renal artery perfusion pressure but also on the balance between afferent and efferent arteriolar tone. In the face of decreased afferent arteriolar pressure or blood flow, low levels of catecholamines, angiotensin, and arginine vasopressin (AVP) induce preferential efferent arteriolar constriction, which maintains glomerular filtration pressure. This adaptive response is reflected by an increase in the calculated filtration fraction (FF), which is the GFR expressed as a fraction of renal plasma flow (RPF): FF = GFR/RPF. High levels of catecholamines and angiotensin (but not AVP) increase afferent arteriolar tone and decrease glomerular filtration pressure (and GFR) out of proportion to RPF, and FF decreases ( Fig. 20-4 ). These mechanisms are described in more detail later.
Tubuloglomerular feedback may be a primary mechanism in renal autoregulation.[2] When the GFR is increased, distal tubular NaCl delivery is enhanced. The increase in chloride is sensed by the macula densa, which triggers the release of renin from the adjacent afferent arteriole. Angiotensin is elaborated and arteriolar constriction ensues, which decreases the GFR.
It may also be a compensatory mechanism to prevent polyuria in acute renal failure ("acute renal success"). When the thick ascending loop becomes ischemic, reabsorption of NaCl ceases, the ability of the tubule to concentrate urine is lost, and theoretically, intractable polyuria should result. Thurau and Boylan [4] suggested that the increased delivery of NaCl to the macula densa triggers angiotensin-mediated arteriolar constriction, which decreases the GFR, induces oliguria, conserves intravascular volume, and protects the organism from dehydration—so-called acute renal success.
Autoregulation enables the kidney to maintain solute and water regulation independently of wide fluctuations in arterial blood pressure. In 1951, the classic dog studies of Shipley and Study[5] demonstrated that the kidney maintains a constant renal blood flow (RBF) and GFR through an arterial pressure range of 80 to 180 mm Hg ( Fig. 20-5 ). It is noteworthy that the urinary flow rate is not subject to autoregulation. Tubular water reabsorption determines the urinary flow rate and is closely related to hydrostatic pressure in the peritubular capillaries. Hypotension, whether induced or inadvertent, results in a decreased urinary flow rate that may be correctable only when arterial blood pressure is restored toward normal.
The precise mechanism of renal autoregulation is not yet defined. Renal vascular resistance appears to be mediated by variable resistance of the preglomerular afferent arteriole. As mean arterial pressure decreases, renal vascular resistance decreases and RBF is maintained. The most plausible explanation is a myogenic response in which the arterioles constrict in response to increased arterial pressure and vice versa. Tubuloglomerular feedback by way of the juxtaglomerular apparatus may also play a role.[6] When arterial pressure increases through the autoregulatory range, it enhances delivery of sodium chloride to the cells of the macula densa, which induces afferent arteriolar constriction and decreased RBF and GFR.[2] The opposite effect occurs when arterial pressure declines.
Though not abolished by most anesthetic agents, autoregulation appears to be impaired in severe sepsis,[7] in acute renal failure,[8] and possibly during cardiopulmonary bypass (CPB).[9] In these situations, RBF declines strikingly during hypotension. It is restored by normalization of renal perfusion pressure, even if such normalization is achieved by vasoconstrictor therapy.
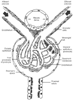 Figure 20-3
The juxtaglomerular apparatus. (From Stanton
BA, Koeppen BM: Elements of renal function. In
Berne RM, Levy MN [eds]: Physiology, 4th ed. St Louis, CV Mosby, 1998, pp 677–698.)
Figure 20-3
The juxtaglomerular apparatus. (From Stanton
BA, Koeppen BM: Elements of renal function. In
Berne RM, Levy MN [eds]: Physiology, 4th ed. St Louis, CV Mosby, 1998, pp 677–698.)
 Figure 20-4
Afferent and efferent arteriolar control mechanisms.
GFP, glomerular filtration pressure. (Redrawn from Sladen RN, Landry D:
Renal blood flow regulation, autoregulation, and vasomotor nephropathy. Anesthesiol
Clin North Am 18:791–807, 2000.)
Figure 20-4
Afferent and efferent arteriolar control mechanisms.
GFP, glomerular filtration pressure. (Redrawn from Sladen RN, Landry D:
Renal blood flow regulation, autoregulation, and vasomotor nephropathy. Anesthesiol
Clin North Am 18:791–807, 2000.)
 Figure 20-5
Autoregulation of the glomerular filtration rate (GFR)
and renal blood flow (RBF), based on the original work of Shipley and Study.[5]
GFR and RBF remain constant between a renal arterial pressure of 80 and 180 mm Hg.
(From Pitts RF: Physiology of the Kidney and Body Fluids. Chicago, Year
Book, 1974.)
Figure 20-5
Autoregulation of the glomerular filtration rate (GFR)
and renal blood flow (RBF), based on the original work of Shipley and Study.[5]
GFR and RBF remain constant between a renal arterial pressure of 80 and 180 mm Hg.
(From Pitts RF: Physiology of the Kidney and Body Fluids. Chicago, Year
Book, 1974.)
|
|
|
|
|
|
|
|
|
|
|
|
|