|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The tubule has four distinct segments: the proximal tubule, the loop of Henle, the distal tubule, and the connecting segment. The loop of Henle itself is divided into the pars recta (the straight portion of the proximal tubule), the descending and ascending thin limb segments, and the thick ascending limb. Each distal tubule drains into a collecting duct, which courses through the cortex, outer medulla, and inner medulla before entering the renal pelvis at the papilla (see Fig. 20-1 ).
Nephrons may be classified as cortical or juxtamedullary nephrons. Cortical nephrons populate the outer and middle renal cortex, are far more numerous, receive about 85% of RBF, and have short loops of Henle. Their efferent arterioles drain into a peritubular capillary plexus. The juxtamedullary nephrons populate the inner renal cortex, receive about 10% of RBF, and have larger glomeruli and long loops of Henle that dive deeply into the inner medulla.[1] Their efferent arterioles drain into elongated vascular conduits, the vasa recta, which are closely applied to the loops of Henle. Although the vasa recta receive less than 1% of RBF, they play an important role in generating the countercurrent mechanism for medullary hypertonicity and renal concentrating ability (see later).
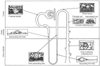 Figure 20-6
Structure-function relationships in the renal tubule.
The most metabolically active components of the tubule are the proximal tubule,
the thick ascending loop of Henle, and the first part of the distal tubule. Their
cells are large, and the capillary surface (basolateral membrane) has many invaginations
rich in mitochondria. The cells of the proximal tubule have a brush border on the
luminal surface (apical cell membrane), whereas the cells of the descending and thin
ascending loops of Henle are flattened with few mitochondria. The second part of
the distal tubule and collecting duct are intermediate in nature. The intercalated
cells of the distal tubule have many mitochondria, whereas the principal cells have
few. (From Stanton BA, Koeppen BM: Elements of renal function. In
Berne RM, Levy MN [eds]: Physiology, 4th ed. St Louis, CV Mosby, 1998, pp 677–698.)
Figure 20-6
Structure-function relationships in the renal tubule.
The most metabolically active components of the tubule are the proximal tubule,
the thick ascending loop of Henle, and the first part of the distal tubule. Their
cells are large, and the capillary surface (basolateral membrane) has many invaginations
rich in mitochondria. The cells of the proximal tubule have a brush border on the
luminal surface (apical cell membrane), whereas the cells of the descending and thin
ascending loops of Henle are flattened with few mitochondria. The second part of
the distal tubule and collecting duct are intermediate in nature. The intercalated
cells of the distal tubule have many mitochondria, whereas the principal cells have
few. (From Stanton BA, Koeppen BM: Elements of renal function. In
Berne RM, Levy MN [eds]: Physiology, 4th ed. St Louis, CV Mosby, 1998, pp 677–698.)
The tubule has an enormous capacity for reabsorption of water and NaCl. Each day, 180 L of protein-free glomerular ultrafiltrate is formed, of which almost 99% of the water and 99% of the sodium is reabsorbed.
Many other filtered substances are completely reabsorbed, but some, such as glucose, have a maximal rate of tubular reabsorption (tubular maximum). Tubular reabsorption of glucose increases at a rate equal to that of the filtered load. If the GFR is constant, the rate is directly proportional to the plasma glucose concentration. Once plasma glucose exceeds the tubular maximum (375 mg/dL), no further glucose is reabsorbed, and glycosuria results. Thereafter, the amount of glucose excreted in urine increases in direct proportion to the filtered load.
Many important endogenous and exogenous solutes are secreted into the tubular lumen from capillary blood. Some also have a tubular maximum for secretion, such as para-aminohippurate (PAH), which is used to calculate RPF. This topic is discussed further in the section "Renal Function Tests."
A striking relationship exists between the structure and function of the different segments of the tubule ( Fig. 20-6 ). The most metabolically active components of the tubule are the proximal tubule, the thick ascending loop of Henle, and the first part of the distal tubule.
Figure 20-7 illustrates a tubular cell in the thick ascending loop of Henle that encompasses all the major mechanisms of reabsorption and secretion. The tubular lumen abuts the apical cell membrane, which joins adjacent cells at the tight junctions. The remainder of the cell is lined by the basolateral cell membrane, which interfaces with the lateral interstitial spaces on either side and with the peritubular capillary at its base. A number of protein-based active transport systems exist, the most important of which is the sodium-potassium-adenosine triphosphatase (Na-K-ATPase) system, situated in the basolateral membrane. It pumps sodium out of the tubular cell into the interstitial fluid (and capillary blood) against a concentration and an electrical gradient in exchange for potassium from inside the tubular cell. The consequent decrease in intracellular sodium concentration facilitates passive reabsorption of sodium from the tubular lumen into the cell. The transport of virtually all solutes is coupled to that of sodium.
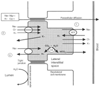 Figure 20-7
Mechanisms of tubular secretion and reabsorption. This
tubular cell in the thick ascending loop of Henle encompasses the major mechanisms
of secretion and reabsorption, one or more of which is used by various segments of
the tubule. The most ubiquitous and important transport mechanism is the energy-requiring
Na-K-ATPase pump in the basolateral cell membrane (1), which pumps sodium out into
the interstitium against its concentration gradient and maintains a low intracellular
concentration. This mechanism favors inward movement of sodium from the tubular
lumen, facilitated by a sodium chloride symporter system on the apical cell membrane
(2), which creates enough potential energy to draw in potassium against its concentration
gradient and is the primary inhibitory site of action of loop diuretics. A sodium-H+
antiporter system on the apical cell membrane (3) aids sodium reabsorption and extrudes
H+
, thereby promoting the reaction of water with carbon dioxide to form
H+
and bicarbonate ion under the influence of carbonic anhydrase (CA).
Bicarbonate diffuses out into the capillary. Sodium reabsorption is thereby coupled
to H+
loss and bicarbonate reabsorption. The transport proteins create
a positive charge in the lumen, which drives ions such as sodium, calcium, potassium,
and magnesium passively through the tight junctions by paracellular diffusion. The
thick ascending loop of Henle is uniquely highly water impermeable, so luminal osmolality
progressively falls to less than 150 mOsm/kg (the "diluting segment"). (Modified
from Stanton BA, Koeppen BM: Elements of renal function. In
Berne RM, Levy MN [eds]: Physiology, 4th ed. St Louis, CV Mosby, 1998, pp 677–698.)
Figure 20-7
Mechanisms of tubular secretion and reabsorption. This
tubular cell in the thick ascending loop of Henle encompasses the major mechanisms
of secretion and reabsorption, one or more of which is used by various segments of
the tubule. The most ubiquitous and important transport mechanism is the energy-requiring
Na-K-ATPase pump in the basolateral cell membrane (1), which pumps sodium out into
the interstitium against its concentration gradient and maintains a low intracellular
concentration. This mechanism favors inward movement of sodium from the tubular
lumen, facilitated by a sodium chloride symporter system on the apical cell membrane
(2), which creates enough potential energy to draw in potassium against its concentration
gradient and is the primary inhibitory site of action of loop diuretics. A sodium-H+
antiporter system on the apical cell membrane (3) aids sodium reabsorption and extrudes
H+
, thereby promoting the reaction of water with carbon dioxide to form
H+
and bicarbonate ion under the influence of carbonic anhydrase (CA).
Bicarbonate diffuses out into the capillary. Sodium reabsorption is thereby coupled
to H+
loss and bicarbonate reabsorption. The transport proteins create
a positive charge in the lumen, which drives ions such as sodium, calcium, potassium,
and magnesium passively through the tight junctions by paracellular diffusion. The
thick ascending loop of Henle is uniquely highly water impermeable, so luminal osmolality
progressively falls to less than 150 mOsm/kg (the "diluting segment"). (Modified
from Stanton BA, Koeppen BM: Elements of renal function. In
Berne RM, Levy MN [eds]: Physiology, 4th ed. St Louis, CV Mosby, 1998, pp 677–698.)
Active transport systems that move solutes in the same direction into or out of the cell are called symporter systems, whereas those that move solutes in opposite directions are called antiporter systems. Solutes are transported by active and passive mechanisms, but water always diffuses passively along an osmotic gradient.
The first part of the proximal tubule reabsorbs about 100% of the filtered glucose, lactate, and amino acids, as well as some phosphate, by coupling with sodium symporter systems.[2] Hydrogen ions are extruded into the tubule by a sodium-H+ antiporter system in exchange for bicarbonate. The absorption of organic anions and bicarbonate in the first part of the proximal tubule results in a relatively high chloride concentration downstream that promotes the passive ingress of chloride. As a consequence, the tubular fluid is positively charged relative to
Most NaCl is absorbed transcellularly by a sodium-H+ and chloride-based antiporter system in the apical cell membrane. The Na-K-ATPase system pumps sodium into the interstitial space, and a potassium-chloride symporter system pumps chloride. The resulting increase in osmolality draws water across as well. In all, about two thirds of the filtered water, chloride, and potassium are reabsorbed by the proximal tubule, coupled with and strongly influenced by sodium absorption.[2]
The proximal tubule is also an important site of secretion of many endogenous anions (bile salts, urate), cations (creatinine, dopamine), and drugs (diuretics, penicillin, probenecid, cimetidine). Organic ions compete with each other for protein transport systems. Thus, administration of probenecid impairs the tubular secretion of penicillin and prolongs its action. In chronic renal insufficiency, organic acids accumulate and compete with drugs such as furosemide for secretor proteins, thereby conferring an apparent "resistance" to loop diuretics.
The metabolically active component of the loop of Henle is the thick ascending loop, which reabsorbs about 20% of the filtered sodium, chloride, potassium, and bicarbonate. Only the descending loop is permeable to water. In the water-impermeable thick ascending loop, sodium is actively reabsorbed, but water remains. In this so-called diluting segment of the kidney, tubular fluid osmolality decreases to less than 150 mOsm/kg H2 O.
As in the proximal tubule, the Na-K-ATPase pump in the basolateral membrane is the engine that drives the resorptive capacity of the thick ascending loop.[2] Sodium moves from the tubular lumen by passive diffusion along its concentration gradient. A sodium-H+ antiporter system in the apical cell membrane mediates the net secretion of H+ and reabsorption of bicarbonate.
An important symporter protein system couples the reabsorption of sodium, chloride, and potassium (the latter against its concentration gradient) across the apical membrane. Blockade of this system is the major mechanism of action of loop diuretics in inhibiting NaCl reabsorption in the thick ascending loop of Henle.
The kidneys receive 20% of the total cardiac output but extract relatively little oxygen. The renal arteriovenous oxygen difference (Δa − ΔvO2 ) is only 1.5 mL/dL. However, there is a marked discrepancy between the renal cortex and medulla with regard to blood flow, oxygen delivery, and oxygen consumption ( Table 20-1 ). The medulla receives only 6% of RBF and has an average oxygen tension (PO2 ) of 8 mm Hg. Thus, it is possible that severe hypoxia could develop in the medulla despite a relatively adequate total RBF, and the metabolically active medullary thick ascending loop of Henle is particularly vulnerable.[10]
The medullary thick ascending loop is also a potential site for
nephrotoxic injury. Intrarenal blood flow is regulated by endogenous vasoactive
compounds. In the
|
|
Cortex | Medulla |
|---|---|---|
| Percent renal blood flow | 94 | 6 |
| Blood flow (mL/min/g) | 5.0 | 0.03 |
| PO2 (mm Hg) | 50 | 8 |
| O2 extraction ratio (VO2 /DO2 ) | 0.18 | 0.79 |
| The renal medulla receives only a small fraction of the total renal blood flow, and flow rates are extremely slow. As a result, tissue oxygen tension is extremely low, and the medulla extracts almost 80% of the oxygen delivered to it. A very mild reduction in total and cortical renal blood flow may therefore induce ischemia and hypoxia in the renal medulla. | ||
| DO2 , oxygen delivery; PO2 , oxygen tension; VO2 , oxygen consumption. | ||
| Data from Brezis M, Rosen S: Hypoxia of the renal medulla—its implications for disease. N Engl J Med 332:647–655, 1995. | ||
Stress (pain, trauma, hemorrhage, hypoperfusion, sepsis, congestive heart failure) activates the sympathoadrenal system and results in renal cortical constriction and potential tubular ischemia. The kidney is relatively devoid of β2 receptors, so epinephrine release induces predominantly vasoconstriction through α receptor or angiotensin activation.
In hemodynamically mediated renal injury, the initial response to renal hypoperfusion is increased active NaCl absorption in the thick ascending limb. Such absorption increases oxygen consumption in the face of decreased oxygen delivery. Subsequent sympathoadrenal responses and renal cortical vasoconstriction may be a compensatory attempt to redistribute blood flow to the medulla. Ultimately, adenosine triphosphate (ATP) stores become depleted and active NaCl reabsorption winds down. This increases the NaCl concentration in tubular fluid reaching the macula densa in the distal tubule and thereby results in angiotensin release and afferent arteriolar constriction (i.e., tubuloglomerular feedback). Teleologically, the resultant decrease in GFR benefits renal oxygen balance by decreasing solute reabsorption and oxygen consumption in the medullary thick ascending loop of Henle. [10]
This hypothesis implies that ischemic or nephrotoxic insults to the renal tubules could be alleviated by the administration of loop diuretics or dopaminergic agents. These drugs inhibit active sodium reabsorption in the thick ascending limb, thereby decreasing oxygen consumption and enhancing tubular oxygen balance.
The proximal segment of the distal tubule is structurally and functionally similar to the thick ascending loop. Sodium reabsorption is mediated by an apical cell membrane NaCl symporter system, which is the site of action of thiazide diuretics.[2]
The last part of the distal tubule is composed of two types of cells. Principal cells reabsorb sodium and water and secrete potassium via the Na-K-ATPase pump, and intercalated cells secrete H+ and reabsorb bicarbonate by means of an H+ -ATPase pump in the apical cell membrane.
|
|
|
|
|
|
|
|
|
|
|
|
|