Pacemaker Indications
Indications for permanent pacing are shown in Table
35-3
and are reviewed in detail elsewhere.[15]
Classically, pacemakers are used to treat patients with diseases of the sinus node
(improper impulse formation) and diseases of the AV node (improper impulse conduction).
Pacing
TABLE 35-2 -- Rate modulation (activity) sensors
|
Approved in the United States
*
|
Under Investigation
*
|
|
Vibration sensor |
Right ventricular stroke volume |
|
Motion sensor |
Blood pH |
|
Minute ventilation (bio-impedance sensor) |
Blood temperature |
|
QT interval (Vitatron only) |
Mixed venous oxygen sensor |
|
Right ventricular pressure (Biotronik only) |
Systolic time intervals |
|
Evoked response |
|
Intracardiac impedance |
*Of
the sensors used to detect exercise in a patient with a cardiac pacemaker, five types
are approved in the United States, and many others are under investigation. Some
devices have two sensors and can be programmed for cross-checking to prevent increases
in heart rate from spurious causes. Minute ventilation sensors are very sensitive
to stray electromagnetic interference, and patients have been inappropriately treated
for pacemaker-driven tachycardias as a result. There is little perioperative experience
with right ventricular pressure sensors. Most pacemaker experts[90]
recommend programming rate modulation to the Off position in the perioperative period
to prevent confusion between an intrinsic tachycardia and a pacemaker-induced tachycardia.
can be used to reduce the outflow tract obstruction in hypertrophic obstructive cardiomyopathy
(HOCM) in both adults and children, since paced ventricular conduction takes place
in a left bundle branch pattern (left ventricular septum depolarizes after the other
segments, rather than as the early systolic event).[16]
[17]
Finally, in August, 2001, devices were approved
by the U.S. FDA for three-chamber pacing (right atrium, both ventricles) to treat
dilated cardiomyopathy (DCM).[18]
To accomplish
left ventricular pacing, a wire is placed through the right atrium and into the coronary
sinus (CS) ( Fig. 35-2
).
Most of the current devices connect the electrodes from the CS lead in parallel
with the electrodes on the RV lead. In the setting of ventricular noncapture, this
parallel connection can lead to ventricular overcounting with resultant inappropriate
antitachycardia therapy for the patient with a DCM pacing defibrillator.[19]
[20]
Devices in clinical trial (as well as the
Reveal
Defibrillator from
TABLE 35-3 -- Indications for pacemaker placement
|
Symptomatic diseases of impulse formation (i.e., sinus node disease)
*
|
|
Symptomatic diseases of impulse conduction (i.e., atrioventricular
nodal disease) |
|
Long QT syndrome |
|
Hypertrophic obstructive cardiomyopathy
†
|
|
Dilated cardiomyopathy (also called cardiac resynchronization
therapy)
†
|
*Indications
for permanent pacing are shown. Most patients with pacemakers fall into the first
two categories listed.
†Requires
100% ventricular pacing to be effective. Short atrioventricular delays (120 to 150
msec) are programmed.
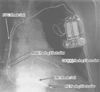 Figure 35-2
A defibrillator system with biventricular (BiV) pacemaker
capability. Three leads are placed: a conventional, bipolar lead to the right atrium;
a quadripolar lead to the right ventricle (RV); and a lead to the coronary sinus
(CS). This system is designed to provide "resynchronization therapy" in the setting
of a dilated cardiomyopathy with a prolonged QRS (and frequently with a prolonged
PR interval). The bipolar lead in the right atrium performs sensing and pacing functions.
Likewise, the bipolar tip in the RV performs pacing and sensing functions. The
lead in the CS depolarizes the left ventricle (LV). In most BiV devices, both ventricles
are depolarized simultaneously, because the electrode on the RV lead are connected
in parallel with the electrode on the CS lead. This parallel connection can cause
ventricular oversensing (and inappropriate antitachycardia therapy) in an implantable
cardioverter-defibrillator (ICD) if the pacemaker fails to depolarize one or both
of the ventricular chambers. The presence of "shock" conductors on the RV lead,
called coils, in the RV and the superior vena cava
(SVC) distinguish a defibrillation system from a conventional pacemaking system.
Typically, the SVC shock coil is electrically identical to the defibrillator case,
called the can. When the defibrillation circuitry
includes the ICD case, it is called an active can configuration.
Figure 35-2
A defibrillator system with biventricular (BiV) pacemaker
capability. Three leads are placed: a conventional, bipolar lead to the right atrium;
a quadripolar lead to the right ventricle (RV); and a lead to the coronary sinus
(CS). This system is designed to provide "resynchronization therapy" in the setting
of a dilated cardiomyopathy with a prolonged QRS (and frequently with a prolonged
PR interval). The bipolar lead in the right atrium performs sensing and pacing functions.
Likewise, the bipolar tip in the RV performs pacing and sensing functions. The
lead in the CS depolarizes the left ventricle (LV). In most BiV devices, both ventricles
are depolarized simultaneously, because the electrode on the RV lead are connected
in parallel with the electrode on the CS lead. This parallel connection can cause
ventricular oversensing (and inappropriate antitachycardia therapy) in an implantable
cardioverter-defibrillator (ICD) if the pacemaker fails to depolarize one or both
of the ventricular chambers. The presence of "shock" conductors on the RV lead,
called coils, in the RV and the superior vena cava
(SVC) distinguish a defibrillation system from a conventional pacemaking system.
Typically, the SVC shock coil is electrically identical to the defibrillator case,
called the can. When the defibrillation circuitry
includes the ICD case, it is called an active can configuration.
Guidant Medical and InSynch III from Medtronic) have separate sensing and output
controls for the ventricular wires.
Pacing for HOCM and DCM requires careful attention to pacer programming.
In order to be effective in these patients, the pacemaker must provide the stimulus
for ventricular depolarization, and AV synchrony must be preserved.[21]
Pacemaker inhibition or loss of pacing (i.e., from native conduction, atrial irregularity,
ventricular irregularity, development of junctional rhythm, or electromagnetic interference)
can lead to deteriorating hemodynamics in these patients.
Biventricular pacing might cause inappropriate lengthening of
the Q-T interval in susceptible patients, and this lengthening has been reported
to be associated with torsades de pointes.[22]
As a result of this report, the prudent anesthesiologist should ensure adequate access
to rapid defibrillation for the patient with biventricular pacing accomplished without
an ICD.
 Figure 35-2
A defibrillator system with biventricular (BiV) pacemaker
capability. Three leads are placed: a conventional, bipolar lead to the right atrium;
a quadripolar lead to the right ventricle (RV); and a lead to the coronary sinus
(CS). This system is designed to provide "resynchronization therapy" in the setting
of a dilated cardiomyopathy with a prolonged QRS (and frequently with a prolonged
PR interval). The bipolar lead in the right atrium performs sensing and pacing functions.
Likewise, the bipolar tip in the RV performs pacing and sensing functions. The
lead in the CS depolarizes the left ventricle (LV). In most BiV devices, both ventricles
are depolarized simultaneously, because the electrode on the RV lead are connected
in parallel with the electrode on the CS lead. This parallel connection can cause
ventricular oversensing (and inappropriate antitachycardia therapy) in an implantable
cardioverter-defibrillator (ICD) if the pacemaker fails to depolarize one or both
of the ventricular chambers. The presence of "shock" conductors on the RV lead,
called coils, in the RV and the superior vena cava
(SVC) distinguish a defibrillation system from a conventional pacemaking system.
Typically, the SVC shock coil is electrically identical to the defibrillator case,
called the can. When the defibrillation circuitry
includes the ICD case, it is called an active can configuration.
Figure 35-2
A defibrillator system with biventricular (BiV) pacemaker
capability. Three leads are placed: a conventional, bipolar lead to the right atrium;
a quadripolar lead to the right ventricle (RV); and a lead to the coronary sinus
(CS). This system is designed to provide "resynchronization therapy" in the setting
of a dilated cardiomyopathy with a prolonged QRS (and frequently with a prolonged
PR interval). The bipolar lead in the right atrium performs sensing and pacing functions.
Likewise, the bipolar tip in the RV performs pacing and sensing functions. The
lead in the CS depolarizes the left ventricle (LV). In most BiV devices, both ventricles
are depolarized simultaneously, because the electrode on the RV lead are connected
in parallel with the electrode on the CS lead. This parallel connection can cause
ventricular oversensing (and inappropriate antitachycardia therapy) in an implantable
cardioverter-defibrillator (ICD) if the pacemaker fails to depolarize one or both
of the ventricular chambers. The presence of "shock" conductors on the RV lead,
called coils, in the RV and the superior vena cava
(SVC) distinguish a defibrillation system from a conventional pacemaking system.
Typically, the SVC shock coil is electrically identical to the defibrillator case,
called the can. When the defibrillation circuitry
includes the ICD case, it is called an active can configuration.