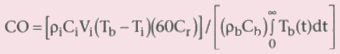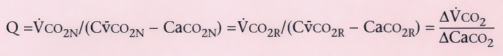APPENDIX 6: Measurement of Cardiac Output by the Thermal
Dilution
and Mass Flow Technique
A commonly used method of measuring blood flow is dilutional calculation,
that is, dye or thermal dilution. These methods are simply mass or energy balances
that determine the volume of fluid that has been diluted by adding a volume of dye
or a given thermal energy. If you have a bucket of water at room temperature (25°C)
and you want to determine the volume of water in the bucket, you could add a known
volume of water at a known temperature. If 100 mL of water at 35°C is added
to the bucket and the final temperature is 27°C, the unknown volume can be
calculated by balancing the heat energy associated with the dilutional process, assuming
that no heat is lost to the environment. This dilutional process can be used to
measure blood flow by completing this same heat balance measured over time. Thermodilution
cardiac output measurements can have significant error as a result of the many assumptions
associated with the technique, such as rapid injection of the thermodilution injectate;
accurate temperature and volume of the injected fluid; constant known heat capacity
of blood, which in reality is a function of the hematocrit value; little heat loss
to the lung; and so on.
Thermistor probes are most commonly used for clinical monitoring
of patient temperature because they are inexpensive, small, and flexible. For these
reasons, the thermistor probe is also used to measure cardiac output determined by
the thermodilution technique. Computation of cardiac output performed in this manner
is, in effect, a heat balance for the right side of the heart. (Heat balance is
a method of accounting for all heat in a process or change involving transfer of
heat.) The technique consists of quick injection of a known volume of a sterile
solution (usually 10 mL of 5% dextrose in water) into the right side of the heart
while a sensor notes the temperature of the blood in the pulmonary artery. It is
assumed that the cold injected solution equilibrates thermally with the blood as
it perfuses the pulmonary artery but that the solution does not acquire heat from
other tissues. The following equation is the solution of this heat balance:
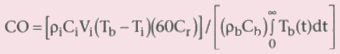
where CO is cardiac output (L/min); ρi
and ρb
are the
densities of the injectate and blood, respectively; Ci
and Cb
are the heat capacities of the injectate and blood, respectively; Vi
is
the volume of the injectate; Tb
and Ti
are the temperature
of the blood and the injectate, respectively; Cr is a computational constant that
corrects for the rising temperature of the injectate; and the integration is the
area under the thermodilution curve. Because the injectate warms as it is injected
through the catheter before mixing with the blood, the correction factor Cr is applied
to the equation.
The same principle of balance used for temperature determination
of cardiac output can be applied to O2
or CO2
balance as well.
Classically, oxygen balance is used as described by Fick. Measurement of oxygen
consumption and content is cumbersome, and a modification of the Fick equation using
CO2
production is also used. NICO is a new partial rebreathing method
using the following equation:
Q = V̇CO2
/(Cv̄CO2
− CaCO2
)
Cardiac output (Q) is simply the expired CO2
divided by the arterial-venous
difference in CO2
. Assuming that Q does not change, the equations for
CO2
elimination must be the same with or without rebreathing, where N
indicates normal breathing and R indicates rebreathing. By rearranging the equations,
cardiac output is the ratio of the change in elimination of CO2
divided
by the change in arterial CO2
content. Arterial CO2
content
is derived from the slope of PETCO2
.
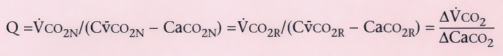
Therefore, we can see that potential errors are induced by violations of the following
assumptions: (1) change in Q during the measurement period, (2) change in metabolic
rate and hence production of CO2
, and (3) change in ventilation.
As a trend analysis, patients with chronic obstructive lung disease,
in which the absolute value of PaCO2
is
widely different from PETCO2
, may have
an absolute error in Q determination by this method, but the relative changes should
track.