Static Pressure Measurement (Manometer)
We have defined pressure as the force acting on a surface per
unit area. A fluid is defined as matter that continuously
deforms (changes shape) as long as any stress (surface force) is applied to it.
Fluids are subdivided into liquids, which are relatively incompressible, and gases,
which are compressible.
Pressure exists and can be measured everywhere within a fluid,
even when it is not in contact with a rigid surface. Although the SI unit of pressure
is the pascal (or newton per meter squared), in medicine we more commonly use units
such as millimeters of mercury (mm Hg) and centimeters of water (cm H2
O).
How does a unit of length become a unit of pressure? This is the principle of the
liquid manometer, the oldest method of pressure measurement. The manometer balances
the pressure to be measured against the pressure exerted by a vertical column of
liquid of known density, for example, mercury and water. The density of a fluid
is its mass per unit volume, which has SI units of kilograms per cubic meter
 Figure 30-10
Potential energy and kinetic energy. Potential energy
is stored energy that can be released or converted into kinetic energy, the energy
of motion. Energy can be stored as gravitational, chemical, electrical, and other
forms of potential energy.
Figure 30-10
Potential energy and kinetic energy. Potential energy
is stored energy that can be released or converted into kinetic energy, the energy
of motion. Energy can be stored as gravitational, chemical, electrical, and other
forms of potential energy.
(kg/m3
) or, commonly, grams per milliliter. The pressure exerted by a
liquid column of height (z) and density (ρ) is simply ρgz (see Appendix
2
for derivation). If the manometer liquid is mercury, which has a density
of 13,600 kg/m3
, the manometer pressure in pascals is
P (Pa) = 13,600 × 9.8 × z (m) = 1.333
× 105
× z (m)
Because these large numbers are rather awkward, we express p in
kilopascals (kPa) and z in millimeters of mercury (mm Hg):
P (kPa) = 0.1333z (mm Hg)
TABLE 30-1 -- Comparison of energy levels of common and uncommon events
|
Event |
Energy |
|
1-kg mass falling 1 m on Earth |
9.8 J |
|
Heartbeat |
10 J (at rest, 60 beats/min, 10 W) |
|
Internal defibrillation for ventricular fibrillation |
30 J |
|
Maximal output of a surface defibrillator |
360 J |
|
1 kcal |
4186 J |
|
Car battery |
1.8 MJ = 1.8 × 106
J |
|
Kilogram of fat |
3.8 × 107
J |
|
Ton of TNT |
4.2 × 109
J |
|
Atomic bomb (Hiroshima) |
15 kilotons = 15 × 103
× 4.2 × 109
J = 6.3 × 1014
J |
|
Hydrogen bomb |
1 megaton = 4.2 × 1015
J |
|
1 kg converted completely to energy |
8.987 × 1016
J |
|
The sun (4.2 × 109
kg matter/sec) |
3.8 × 1026
J/sec |
|
Modified from Hecht E: Physics: Algebra/Trig. Pacific
Grove, CA, Brooks/Cole, 1994. |
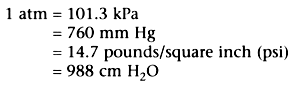
A useful reference for the various pressure units in use today
is the pressure of the earth's atmosphere at sea level, called "one atmosphere" or
1 atm:
For slowly changing pressures, a water or mercury manometer is
simple and dependable ( Fig 30-11
).
The manometer cannot respond quickly to rapid changes in pressure because of its
inertia; that is, the mass of the liquid column resists rapid changes in height.
If a fluid-filled catheter is connected to a patient, the height of the fluid in
the manometer determines the mean pressure at the tip of the catheter. If the pressure
measured is central venous pressure, we can use these data to infer right ventricular
preload. Because this manometer is in direct continuity with the patient's circulation,
the manometer fluid must be compatible with blood; that is, it must be iso-osmolar,
as well as nontoxic.
 Figure 30-10
Potential energy and kinetic energy. Potential energy
is stored energy that can be released or converted into kinetic energy, the energy
of motion. Energy can be stored as gravitational, chemical, electrical, and other
forms of potential energy.
Figure 30-10
Potential energy and kinetic energy. Potential energy
is stored energy that can be released or converted into kinetic energy, the energy
of motion. Energy can be stored as gravitational, chemical, electrical, and other
forms of potential energy.