ALTERATIONS IN ANESTHETIC REQUIREMENT PERTINENT TO
THEORIES OF NARCOSIS
Effects of Temperature
In mammals, the MAC decreases with decreasing body temperatures
for all anesthetics, but the reduction (about 2% to 5%) per degree decrease in body
temperature varies slightly from agent to agent.[5]
Although the gas-phase potencies of inhaled agents increase with decreasing temperature,
there is an associated increase in anesthetic solubility with a decrease in temperature
so that the aqueous-phase potencies remain relatively constant with changes in temperature
( Fig. 4-3
).[12]
For in vitro experiments involving clinical
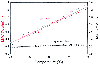 Figure 4-3
Plot of the halothane minimum alveolar concentration
(MAC) in dogs versus temperature. When measured as a function of the gas-phase partial
pressure, MAC decreases with decreasing temperature. When measured as a function
of calculated aqueous concentrations, MAC remains relatively constant with changes
in temperature from 28°C to 43°C. (From Franks NP, Lieb WR: Temperature
dependence of the potency of volatile general anesthetics. Anesthesiology 84:716,
1996.)
Figure 4-3
Plot of the halothane minimum alveolar concentration
(MAC) in dogs versus temperature. When measured as a function of the gas-phase partial
pressure, MAC decreases with decreasing temperature. When measured as a function
of calculated aqueous concentrations, MAC remains relatively constant with changes
in temperature from 28°C to 43°C. (From Franks NP, Lieb WR: Temperature
dependence of the potency of volatile general anesthetics. Anesthesiology 84:716,
1996.)
volatile anesthetics, aqueous concentrations equivalent to 1 MAC range from about
200 to 600 µM.[12]
 Figure 4-3
Plot of the halothane minimum alveolar concentration
(MAC) in dogs versus temperature. When measured as a function of the gas-phase partial
pressure, MAC decreases with decreasing temperature. When measured as a function
of calculated aqueous concentrations, MAC remains relatively constant with changes
in temperature from 28°C to 43°C. (From Franks NP, Lieb WR: Temperature
dependence of the potency of volatile general anesthetics. Anesthesiology 84:716,
1996.)
Figure 4-3
Plot of the halothane minimum alveolar concentration
(MAC) in dogs versus temperature. When measured as a function of the gas-phase partial
pressure, MAC decreases with decreasing temperature. When measured as a function
of calculated aqueous concentrations, MAC remains relatively constant with changes
in temperature from 28°C to 43°C. (From Franks NP, Lieb WR: Temperature
dependence of the potency of volatile general anesthetics. Anesthesiology 84:716,
1996.)